Bioactive Polysaccharides Prevent Lipopolysaccharide-Induced Intestinal Inflammation via Immunomodulation, Antioxidant Activity, and Microbiota Regulation
- PMID: 40807512
- PMCID: PMC12346005
- DOI: 10.3390/foods14152575
Bioactive Polysaccharides Prevent Lipopolysaccharide-Induced Intestinal Inflammation via Immunomodulation, Antioxidant Activity, and Microbiota Regulation
Abstract
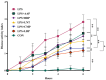
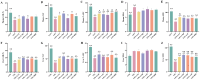
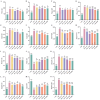
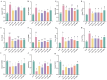
Intestinal inflammation involves barrier impairment, immune hyperactivation, and oxidative stress imbalance. Bioactive polysaccharides universally alleviate inflammation via anti-inflammatory, antioxidant, and microbiota-modulating effects, yet exhibit distinct core mechanisms. Elucidating these differences is vital for targeted polysaccharide applications. This research examines distinct regulatory pathways through which diverse bioactive polysaccharides mitigate lipopolysaccharide-triggered intestinal inflammation in male Kunming (KM) mice. This experiment employed Lentinula edodes polysaccharide (LNT), Auricularia auricula polysaccharide (AAP), Cordyceps militaris polysaccharide (CMP), Lycium barbarum polysaccharide (LBP), and Brassica rapa polysaccharide (BRP). The expression levels of biomarkers associated with the TLR4 signaling pathway, oxidative stress, and intestinal barrier function were quantified, along with comprehensive gut microbiota profiling. The results showed that all five polysaccharides alleviated inflammatory responses in mice by inhibiting inflammatory cytokine release, reducing oxidative damage, and modulating gut microbiota, but their modes of action differed: LBP significantly suppressed the TLR-4/MyD88 signaling pathway and its downstream pro-inflammatory cytokine expression, thereby blocking inflammatory signal transduction and reducing oxidative damage; LNT and CMP enhanced the body's antioxidant capacity by increasing antioxidant enzyme activities and decreasing malondialdehyde (MDA) levels; AAP and BRP enriched Akkermansia (Akk.) within the Verrucomicrobia (Ver.) phylum, upregulating tight junction protein expression to strengthen the intestinal mucosal barrier and indirectly reduce oxidative damage. This research demonstrates that different polysaccharides alleviate inflammation through multi-target synergistic mechanisms: LBP primarily inhibits inflammatory pathways; AAP and BRP focus on intestinal barrier protection and microbiota modulation; and LNT and CMP exert effects via antioxidant enzyme activation. These data support designing polysaccharide blends that leverage complementary inflammatory modulation mechanisms.
Keywords: IBD; NF-κB/MyD88 signaling pathway; gut microbiota; intestinal barrier; oxidative stress; prebiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Combination of Withania coagulans and Fagonia cretica ameliorates hyperuricemia by re-modulating gut microbiota-derived spermidine and traumatic acid.Phytomedicine. 2025 Sep;145:157079. doi: 10.1016/j.phymed.2025.157079. Epub 2025 Jul 17. Phytomedicine. 2025. PMID: 40712280
-
Music intervention mitigates LPS-induced gut barrier disruption and immune stress in broilers via TLR4/NF-κB regulation.Poult Sci. 2025 Jul;104(7):105189. doi: 10.1016/j.psj.2025.105189. Epub 2025 Apr 22. Poult Sci. 2025. PMID: 40294553 Free PMC article.
-
Intestinal inflammation and microbiota modulation impact cochlear function: emerging insights in gut-ear axis.Cell Commun Signal. 2025 Jul 26;23(1):357. doi: 10.1186/s12964-025-02338-1. Cell Commun Signal. 2025. PMID: 40713718 Free PMC article.
-
Immunomodulatory potential of dietary soybean-derived saponins.J Anim Sci. 2024 Jan 3;102:skae349. doi: 10.1093/jas/skae349. J Anim Sci. 2024. PMID: 39529449 Review.
-
Microbiota-dependent modulation of intestinal anti-inflammatory CD4+ T cell responses.Semin Immunopathol. 2025 Apr 1;47(1):23. doi: 10.1007/s00281-025-01049-6. Semin Immunopathol. 2025. PMID: 40167791 Review.
References
-
- Tinazzi M., Sacilotto A., Cocetta V., Giacomini I., Raso F., Bulferi G., De Togni H., Lanza R., Consolo P., Berretta M., et al. Bowel Inflammation and Nutrient Supplementation: Effects of a Fixed Combination of Probiotics, Vitamins, and Herbal Extracts in an In Vitro Model of Intestinal Epithelial Barrier Dysfunction. Yale J. Biol. Med. 2024;97:297–308. doi: 10.59249/JOMF5336. - DOI - PMC - PubMed
-
- Gold S.L., Raman M. Malnutrition Assessment in Patients with Inflammatory Bowel Disease. Can. Ibd Today. 2023;1:32–38. doi: 10.58931/cibdt.2023.119. - DOI
-
- Hyder C.S., Uddin M.A., Jalal M.T., Karim S.S., Taher M.A., Nahar K., Hossain M.S., Sheikh M.S.H., Islam M.S. Frequency and Pattern of Premalignant and Malignant Lesions among Fecal Immunochemical Test Positive Patients. Mymensingh Med. J. 2025;34:745–751. - PubMed
-
- Patel R., Roy V., Kunhipurayil S., Patel K.M., Elassar S., Sojitra V., Desai H.D. Trends of Inflammatory Bowel Disease Burden in the United States (1990–2019), Projections of Deaths to 2040: Insights from the 2019 Global Burden of Disease Study. Inflamm. Bowel Dis. 2024;30:S41–S42. doi: 10.1093/ibd/izae020.086. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

