Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols
- PMID: 40807563
- PMCID: PMC12346670
- DOI: 10.3390/foods14152626
Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols
Abstract
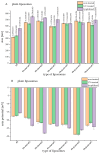
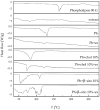
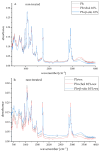
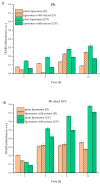
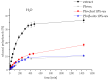
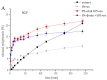
This study developed phospholipid-based liposomes loaded with extract from wild thyme (Thymus serpyllum L.) tea processing residues to enhance polyphenol stability and delivery. Liposomes were prepared with phospholipids alone or combined with 10-30 mol% cholesterol or β-sitosterol. The effect of different lipid compositions on encapsulation efficiency (EE), particle size, polydispersity index (PDI), zeta potential, stability, thermal properties, diffusion coefficient, and diffusion resistance of the liposomes was investigated. Liposomes with 10 mol% sterols (either cholesterol or β-sitosterol) exhibited the highest EE of polyphenols, while increasing sterol content to 30 mol% resulted in decreased EE. Particle size and PDI increased with sterol content, while liposomes prepared without sterols showed the smallest vesicle size. Encapsulation of the extract led to smaller liposomal diameters and slight increases in PDI values. Zeta potential measurements revealed that sterol incorporation enhanced the surface charge and stability of liposomes, with β-sitosterol showing the most pronounced effect. Stability testing demonstrated minimal changes in size, PDI, and zeta potential during storage. UV irradiation and lyophilization processes did not cause significant polyphenol leakage, although lyophilization slightly increased particle size and PDI. Differential scanning calorimetry revealed that polyphenols and sterols modified the lipid membrane transitions, indicating interactions between extract components and the liposomal bilayer. FT-IR spectra confirmed successful integration of the extract into the liposomes, while UV exposure did not significantly alter the spectral features. Thiobarbituric acid reactive substances (TBARS) assay demonstrated the extract's efficacy in mitigating lipid peroxidation under UV-induced oxidative stress. In contrast, liposomes enriched with sterols showed enhanced peroxidation. Polyphenol diffusion studies showed that encapsulation significantly delayed release, particularly in sterol-containing liposomes. Release assays in simulated gastric and intestinal fluids confirmed controlled, pH-dependent polyphenol delivery, with slightly better retention in β-sitosterol-enriched systems. These findings support the use of β-sitosterol- and cholesterol-enriched liposomes as stable carriers for polyphenolic compounds from wild thyme extract, as bioactive antioxidants, for food and nutraceutical applications.
Keywords: Thymus serpyllum; cholesterol; liposomes; proliposome method; β-sitosterol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Jarić S., Mitrović M., Karadžić B. Plant resources used in Serbian medieval medicine. Genet. Resour. Crop Evol. 2014;61:1359–1379. doi: 10.1007/s10722-014-0118-1. - DOI
-
- Jovanović A., Vajić U.-J., Mijin D., Zdunić G., Šavikin K., Branković S., Kitić D., Bugarski B. Polyphenol extraction in microwave reactor using by-product of Thymus serpyllum L. and biological potential of the extract. J. Appl. Res. Med. Aromat. Plants. 2022;31:100417. doi: 10.1016/j.jarmap.2022.100417. - DOI
-
- Brown da Rocha C., Noreña C. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020;16:20190191. doi: 10.1515/ijfe-2019-0191. - DOI
LinkOut - more resources
Full Text Sources

