Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice
- PMID: 40807600
- PMCID: PMC12346151
- DOI: 10.3390/foods14152662
Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice
Abstract
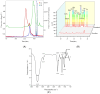
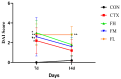
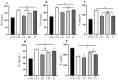
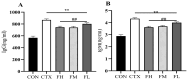
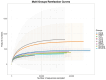
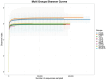
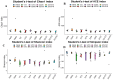
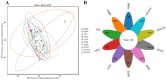
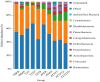
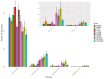
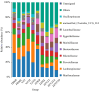
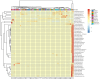
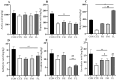
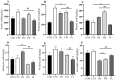
Recent studies indicate that fucoidan may play a crucial role in the metabolism and biological function of the intestinal flora. This study investigates the therapeutic potential of kelp fucoidan on the gut microbiota and immune homeostasis of cyclophosphamide-induced immunosuppressed mice. An immunosuppressive mouse model was established using cyclophosphamide, followed by administration of various kelp fucoidan doses (low-dose fucoidan: 50 mg/(kg·bw)/d, medium-dose fucoidan: 100 mg/(kg·bw)/d, and high-dose fucoidan: 150 mg/(kg·bw)/d) to the experimental groups. Changes in the gut microbiota structure were analyzed using 16S rRNA high-throughput sequencing, alongside simultaneous measurement of serum immune indicators and levels of short-chain fatty acids (SCFAs). Results indicate that kelp fucoidan significantly improved the thymus and spleen indices in immunosuppressed mice (p < 0.05) and elevated serum levels of IgM, IgG and IL-4. Post-kelp fucoidan intervention, there was significant alteration in microbiota ecosystem restructuring, such as proliferation in probiotics, including Lactobacillus and Bifidobacterium, while opportunistic pathogens, such as Enterococcus and Escherichia coli, decreased. Furthermore, the levels of acetic, propionic, and butyric acids in the colonic contents of the kelp fucoidan group significantly improved (p < 0.01). This research demonstrates that kelp fucoidan enhances immune function in immunosuppressed mice by modulating gut microbiota balance and promoting short-chain fatty acid production.
Keywords: immunosuppressed mice; intestinal flora; kelp fucoidan; short chain fatty acid.
Conflict of interest statement
Author Zhengpeng Wei is employed by the company Rongcheng Taixiang Food Co., Ltd., Rongcheng, Shandong. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Milk-processed Polygonatum cyrtonema Hua ameliorates cyclophosphamide-induced immunosuppression in mice by regulating gut microbiota and immune response.Phytomedicine. 2025 Sep;145:157076. doi: 10.1016/j.phymed.2025.157076. Epub 2025 Jul 14. Phytomedicine. 2025. PMID: 40684494
-
BTVT ameliorates offspring blood-brain barrier damage induced by prenatal and lactational neodymium oxide exposure via the gut-brain axis.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2025 Apr 28;50(4):615-624. doi: 10.11817/j.issn.1672-7347.2025.250173. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40785675 Free PMC article. Chinese, English.
-
Integrative multi-omics and bioinformatics analysis of the effects of BaiRui YuPingFeng Powder on intestinal health in broilers.Front Vet Sci. 2025 Jun 18;12:1606531. doi: 10.3389/fvets.2025.1606531. eCollection 2025. Front Vet Sci. 2025. PMID: 40607343 Free PMC article.
-
Role of the intestinal flora-immunity axis in the pathogenesis of rheumatoid arthritis-mechanisms regulating short-chain fatty acids and Th17/Treg homeostasis.Mol Biol Rep. 2025 Jun 21;52(1):617. doi: 10.1007/s11033-025-10714-w. Mol Biol Rep. 2025. PMID: 40544212 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

