Optimizing the Bioprocesses of Bacteriocin Production in Lacticaseibacillus paracasei HD1.7 by the "Acetate Switch": Novel Insights into the Labor Division Between Energy Metabolism, Quorum Sensing, and Acetate
- PMID: 40807629
- PMCID: PMC12346395
- DOI: 10.3390/foods14152691
Optimizing the Bioprocesses of Bacteriocin Production in Lacticaseibacillus paracasei HD1.7 by the "Acetate Switch": Novel Insights into the Labor Division Between Energy Metabolism, Quorum Sensing, and Acetate
Abstract
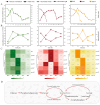
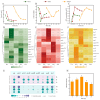
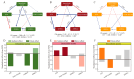
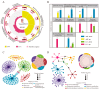
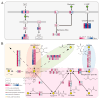
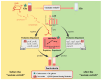
Acetate may act as a signaling molecule, regulating Paracin 1.7 production via quorum sensing (QS) in Lacticaseibacillus paracasei HD1.7. The "acetate switch" phenomenon requires mechanistic exploration to optimize Paracin 1.7 production. The "acetate switch" phenomenon delays with higher glucose levels (30 h, 36 h, and 96 h). Before the occurrence of the "acetate switch", the ATP content increases and peaks at the "acetate switch" point and the NAD+/NADH ratio decreases, indicating energy changes. Moreover, the QS genes used for the pre-regulation of bacteriocin, such as prcKR, comCDE, were highly expressed. After the "acetate switch", the ATP content decreased and the QS genes for the post-regulation of bacteriocin were highly expressed, such as rggs234 and sigma70-1/70-2. The "acetate switch" could act as an energy switch, regulating bacterial growth and QS genes. Before and after the "acetate switch", some metabolic pathways were significantly altered according to the transcriptomic analysis by HD1.7 and HD1.7-Δpta. In this study, acetate was used as an input signal to regulate the two-component system, significantly influencing the bacteriocin expression system. And this study clarifies the roles of acetate, energy, and quorum sensing in promoting Paracin 1.7 production, providing a theoretical basis for optimizing the bacteriocin fermentation process of HD1.7.
Keywords: Lacticaseibacillus paracasei HD1.7; acetate switch; bioprocess; quorum sensing; transcriptomic analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Unveiling the molecular mechanisms of human platelet lysate in enhancing endometrial receptivity.Hum Reprod. 2025 Jul 15:deaf118. doi: 10.1093/humrep/deaf118. Online ahead of print. Hum Reprod. 2025. PMID: 40663777
-
Mapping of quorum sensing interaction network of commensal and pathogenic staphylococci.mBio. 2025 Aug 13;16(8):e0096725. doi: 10.1128/mbio.00967-25. Epub 2025 Jul 16. mBio. 2025. PMID: 40667988 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
Grants and funding
- PL2024D015/Heilongjiang Provincial Natural Science Foundation of China
- RC2024DY192/Harbin Science and Technology Innovation Talent Project
- HST2022TR004/Scientific Research Project of Ecological Environment Protection of Heilongjiang Provincial Department of Ecological Environment
- 2023-KYYWF-1448/Basic Research Operating Costs of Colleges and Universities in Heilongjiang Province
- No. 32071519/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

