Oxidized Phospholipids on ApoB-100, Platelet Activation and Reactivity, and Long-Term Cardiovascular Outcomes
- PMID: 40808656
- PMCID: PMC12421647
- DOI: 10.1161/ATVBAHA.125.322347
Oxidized Phospholipids on ApoB-100, Platelet Activation and Reactivity, and Long-Term Cardiovascular Outcomes
Abstract
Background: OxPL-apoB (oxidized phospholipids [OxPL] on apoB-100), which include OxPL present on Lp(a) (lipoprotein[a]), are associated with higher cardiovascular risk. Experimental studies suggest that OxPL may influence platelet function.
Methods: This observational study assessed the association of OxPL-apoB with intrinsic and on‑clopidogrel platelet reactivity and long-term cardiovascular events in patients undergoing coronary angiography with or without percutaneous coronary intervention in 2040 patients in the EXCELSIOR trial (Impact of Extent of Clopidogrel-Induced Platelet Inhibition During Elective Stent Implantation on Clinical Event Rate). The association of OxPL-apoB to expression of CD62P, CD41, or PAC-1 levels and intrinsic and on-clopidogrel platelet reactivity to collagen and ADP was determined. The relationship of OxPL-apoB and Lp(a) to myocardial infarction-free survival and all-cause mortality at a median of 7 years was assessed using Cox regression models.
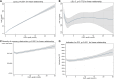
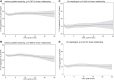
Results: Elevated levels of OxPL-apoB were associated with the severity of coronary obstruction, and higher prevalence of prior myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft surgery. No significant associations were present between OxPL-apoB and intrinsic or on-clopidogrel platelet reactivity or activation of platelet receptors. Analyzed individually in separate multivariable models, both OxPL-apoB (hazard ratio, 1.022 [95% CI, 1.005-1.040]; P=0.010) and Lp(a) (hazard ratio, 1.002 [95% CI, 1.000-1.005]; P=0.032) were associated with worse myocardial infarction-free survival. However, in a joint multivariable model analyzed together, neither OxPL-apoB nor Lp(a) was significant. The optimal cut point for myocardial infarction-free survival for OxPL-apoB was 8 nmol/L (hazard ratio, 1.391 [95% CI, 1.086-1.780]; P=0.009) and for Lp(a) 30 mg/dL (hazard ratio, 1.261 [95% CI, 1.012-1.570]; P=0.038).
Conclusions: In patients undergoing coronary angiography with or without percutaneous coronary intervention, OxPL-apoB was not associated with intrinsic and on-clopidogrel platelet reactivity mediated by collagen or ADP. The association of OxPL-apoB and Lp(a) suggests that the accumulation of OxPL on Lp(a) may be a key determinant of long-term cardiovascular outcomes.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT00457236.
Keywords: aspirin; atherosclerosis; clopidogrel; lipoproteins; myocardial infarction.
Conflict of interest statement
S. Tsimikas is a co-inventor and has received royalties from patents owned by the University of California-San Diego. S. Tsimikas is a cofounder of and has an equity interest in Oxitope and Kleanthi Diagnostics and has a dual appointment at the University of California San Diego and Ionis Pharmaceuticals. A. Kille reports speaker honoraria from Sysmex. T. Nührenberg is an employee of Novartis as of October 1, 2021. W. Hochholzer received consultancy fees or speaker honoraria from Daiichi Sankyo, Novartis, and Bayer. F.-J. Neumann reports that his institution has received research grants, consultancy fees, and speaker honoraria from Daiichi Sankyo, Astra Zeneca, Sanofi-Aventis, Bayer, The Medicines Company, Bristol, Novartis, Roche, Boston Scientific, Biotronik, Medtronic, Edwards, and Ferrer. W. Hochholzer received consultancy honoraria from Daiichi Sankyo, The Medicines Company, and Ferrer and reports speaker honoraria from AstraZeneca, Bayer Vital, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, and feo GmbH as well as research grants paid to his former institution from German Heart Foundation, Novartis, and Sysmex. The other authors report no conflicts.
Figures



References
-
- Tsimikas S, Witztum JL. Oxidized phospholipids in cardiovascular disease. Nat Rev Cardiol. 2024;21:170–191. doi: 10.1038/s41569-023-00937-4 - PubMed
-
- Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, Moriarty PM, Rader DJ, Remaley AT, Reyes-Soffer G, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71:177–192. doi: 10.1016/j.jacc.2017.11.014 - PMC - PubMed
-
- Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925–3946. doi: 10.1093/eurheartj/ehac361 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

