Shikonin as a therapeutic agent in renal cell carcinoma: insights from TEK-related causal association with glaucoma
- PMID: 40808675
- PMCID: PMC12343566
- DOI: 10.3389/fphar.2025.1580704
Shikonin as a therapeutic agent in renal cell carcinoma: insights from TEK-related causal association with glaucoma
Abstract
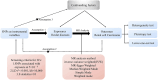
Introduction: Renal cell carcinoma (RCC) is a lethal malignancy with rising incidence, while glaucoma, a chronic eye disease, shares systemic mechanisms such as oxidative stress and inflammation with cancers. This study aimed to investigate the causal link between glaucoma and RCC and explore molecular intersections to identify novel therapeutic targets.
Methods: A two-step Mendelian randomization (MR) analysis using genetic data from the NHGRI-EBI GWAS Catalog and FinnGen database was performed, supplemented by NHANES data. Gene expression analysis (GSE53757, E-MTAB-1980) identified glaucoma-related genes in RCC. Molecular docking and functional assays evaluated shikonin's effects on TEK and AKT/mTOR signaling.
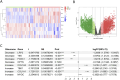
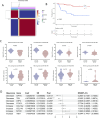
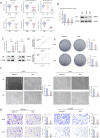
Results: MR revealed a significant causal relationship between glaucoma and RCC. TEK, a glaucoma-related gene, was downregulated in RCC tissues and correlated with advanced tumor stage and metastasis. Shikonin and acetylshikonin upregulated TEK expression, inhibited RCC cell proliferation/migration, and suppressed AKT/mTOR phosphorylation.
Discussion: These findings support a role for glaucoma-associated genes in RCC development and progression, highlighting shikonin as a promising therapeutic agent targeting this molecular axis.
Keywords: Mendelian randomization (MR); NHANES; TEK; gene expression; glaucoma; renal cell carcinoma (RCC); shikonin.
Copyright © 2025 Jia, Liang, Zou, Li, Chen, Zhang, Bian and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Genetic association of lipid traits and lipid-related drug targets with normal tension glaucoma: a Mendelian randomization study for predictive preventive and personalized medicine.EPMA J. 2024 Jul 13;15(3):511-524. doi: 10.1007/s13167-024-00373-5. eCollection 2024 Sep. EPMA J. 2024. PMID: 39239107
-
Unraveling the Molecular Nexus between Ankylosing Spondylitis and IgA Nephropathy: Insights from Mendelian Randomization and Bioinformatics Analysis.Nephron. 2025;149(8):446-462. doi: 10.1159/000544970. Epub 2025 Mar 12. Nephron. 2025. PMID: 40073855
-
mTOR and mTOR phosphorylation status in primary and metastatic renal cell carcinoma tissue: differential expression and clinical relevance.J Cancer Res Clin Oncol. 2019 Jan;145(1):153-163. doi: 10.1007/s00432-018-2775-5. Epub 2018 Oct 27. J Cancer Res Clin Oncol. 2019. PMID: 30368665 Free PMC article.
-
Comprehensive assessment of glaucoma in patients with high myopia: a systematic review and meta-analysis with a discussion of structural and functional imaging modalities.Int Ophthalmol. 2024 Oct 11;44(1):405. doi: 10.1007/s10792-024-03321-4. Int Ophthalmol. 2024. PMID: 39392516 Free PMC article.
-
Insights from Mendelian randomization and genetic correlation analyses into the relationship between endometriosis and its comorbidities.Hum Reprod Update. 2023 Sep 5;29(5):655-674. doi: 10.1093/humupd/dmad009. Hum Reprod Update. 2023. PMID: 37159502 Free PMC article. Review.
References
-
- Bahadoram S., Davoodi M., Hassanzadeh S., Bahadoram M., Barahman M., Mafakher L. (2022). Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 39 (3), 2022-vol3. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

