Identification of potential drug-induced neuralgia signals through disproportionality analysis of the FAERS database
- PMID: 40808677
- PMCID: PMC12343520
- DOI: 10.3389/fphar.2025.1645114
Identification of potential drug-induced neuralgia signals through disproportionality analysis of the FAERS database
Abstract
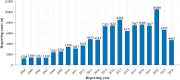
Background: Drug-induced neuralgia is a common and significant adverse reaction. This study analyzed the United States food and drug administration adverse event reporting system (FAERS) database (2004-2024) to identify relevant drugs and potential mechanisms.
Methods: We conducted an association analysis between drugs and neuralgia using the FAERS database. Disproportionality analysis methods, including the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and empirical Bayesian geometric mean (EBGM), were applied. Data from 2004 to 2024 were analyzed to identify drugs potentially associated with neuralgia.
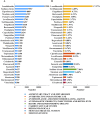
Results: Among the 103,678 reports of neuralgia-related adverse events, 60.29% involved female patients, and 30.40% were aged between 41 and 64 years. The most common underlying medical conditions were plasma cell myeloma (14.28%) and multiple sclerosis (10.65%). The analysis revealed significant associations between neuralgia and several classes of drugs, including chemotherapeutic agents, certain antibiotics, and immunosuppressants, potentially attributable to neurotoxicity, immune-mediated mechanisms, or metabolic disruptions. Notably, lenalidomide exhibited the strongest association with neuralgia, followed by sodium citrate. These findings underscore the importance of early recognition, safer prescribing strategies, and further investigation to mitigate neurotoxic risks.
Conclusion: This study identifies key drugs, including chemotherapeutics, antibiotics, and immunosuppressants, associated with drug-induced neuralgia through FAERS data analysis, highlighting the need for early detection, safer prescribing practices, and further research into mitigating neurotoxicity.
Keywords: FAERS database; adverse drug event; neuralgia; neurotoxicity; signal detection.
Copyright © 2025 An, Zheng, Jiang, Meng, Yin and An.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system.Sci Rep. 2025 Aug 7;15(1):28886. doi: 10.1038/s41598-025-13786-7. Sci Rep. 2025. PMID: 40775011 Free PMC article.
-
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024.Front Pharmacol. 2025 Jun 9;16:1516449. doi: 10.3389/fphar.2025.1516449. eCollection 2025. Front Pharmacol. 2025. PMID: 40552159 Free PMC article.
-
Stratified analysis of the association between anti-obesity medications and digestive adverse events: a real-world study based on the FDA adverse event reporting system database.BMC Pharmacol Toxicol. 2024 Sep 12;25(1):64. doi: 10.1186/s40360-024-00789-9. BMC Pharmacol Toxicol. 2024. PMID: 39267168 Free PMC article.
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
Gastrointestinal stromal tumors with the use of ripretinib and sunitinib: real-world adverse event analysis based on the FDA adverse event reporting system (FAERS).Front Pharmacol. 2025 Jun 26;16:1561937. doi: 10.3389/fphar.2025.1561937. eCollection 2025. Front Pharmacol. 2025. PMID: 40642018 Free PMC article. Review.
References
-
- Baah E. M. (2020). Analysis of data on adverse drug events reported to the food and drugs administration of the United States of America. Open J. Statistics 10 (2), 203–227. 10.4236/ojs.2020.102015 - DOI
