Enhanced NIR-II Nanoparticle Probe for PSMA-Targeted Molecular Imaging and Prostate Cancer Diagnosis
- PMID: 40808713
- PMCID: PMC12345944
- DOI: 10.2147/IJN.S532080
Enhanced NIR-II Nanoparticle Probe for PSMA-Targeted Molecular Imaging and Prostate Cancer Diagnosis
Abstract
Introduction: Prostate-specific membrane antigen (PSMA) is a well-established biomarker overexpressed in prostate cancer (PCa). However, existing PSMA-targeted imaging probes suffer from radiation exposure, limited tissue penetration, and inadequate intraoperative performance. To overcome these challenges, we developed a novel near-infrared-II (NIR-II) fluorescent nanoprobe for both in vivo and ex vivo imaging of PCa.
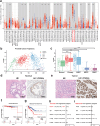
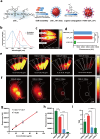
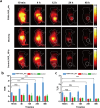
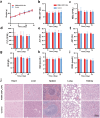
Methods: An organic semiconducting polymer (OSP12) with strong NIR-II fluorescence and excellent photostability was self-assembled into nanoparticles (NPs) using DSPE-PEG-Mal. These OSP12 NPs were then conjugated with ACUPA-SH, (S)-2-[3-((S)-5-amino-1-carboxypentyl)ureido]pentanedioic acid, a thiol-modified glutamate-urea-lysine derivative that specifically targets PSMA, via a maleimide-thiol click reaction to form PSMA-OSP12 NPs. The probe's targeting specificity was assessed using PSMA positive and negative cell lines under NIR-II imaging. For in vivo evaluation, subcutaneous xenograft tumors were established in BALB/c nude mice. Animals were randomly assigned to PSMA-OSP12 NP, blocking (ACUPA pre-injection), and control (OSP12 NPs) groups (n = 3 per group). Ex vivo tumor slice imaging was performed on fresh tissue sections. Biosafety was evaluated in healthy mice (n=5) through hematological, biochemical, and histopathological analyses.
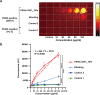
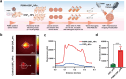
Results: PSMA-OSP12 NPs exhibited excellent optical properties in the NIR-II window, including high photostability, negligible autofluorescence, and deep tissue penetration. In vitro assays confirmed selective binding to PSMA-positive cells, while in vivo imaging demonstrated sustained tumor accumulation with a peak TBR of 7.40 ± 1.28 at 48 h post-injection. This performance significantly surpassed OTL78 and Cy-KUE-OA, enabling flexible surgical planning and real-time intraoperative guidance. In ex vivo tissue imaging, PSMA-OSP12 NPs provided high-contrast tumor delineation without systemic administration. Biosafety evaluations revealed no significant systemic toxicity, and biodistribution analysis indicated hepatic metabolism and biliary clearance.
Conclusion: PSMA-OSP12 NPs are a promising NIR-II fluorescent probe with excellent tumor specificity, deep tissue imaging capability, and good biocompatibility, supporting their application in fluorescence-guided surgery and ex vivo tumor evaluation.
Keywords: NIR-II; fluorescence-guided surgery; molecular imaging; nanoparticles; organic semiconducting polymer; prostate cancer; prostate-specific membrane antigen; tumor targeting.
© 2025 Jiang et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Synthesis and Preliminary Biological Assessment of Carborane-Loaded Theranostic Nanoparticles to Target Prostate-Specific Membrane Antigen.ACS Appl Mater Interfaces. 2021 Nov 24;13(46):54739-54752. doi: 10.1021/acsami.1c16383. Epub 2021 Nov 9. ACS Appl Mater Interfaces. 2021. PMID: 34752058
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Evaluation of 134Ce/134La-PSMA-617 for PET Imaging and Auger Electron Therapy of Prostate Cancer.J Nucl Med. 2025 Aug 1;66(8):1252-1257. doi: 10.2967/jnumed.125.269751. J Nucl Med. 2025. PMID: 40506238 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Near-Infrared-II Fluorescent Probes for Analytical Applications: From In Vitro Detection to In Vivo Imaging Monitoring.Acc Chem Res. 2025 Feb 18;58(4):543-554. doi: 10.1021/acs.accounts.4c00671. Epub 2025 Feb 5. Acc Chem Res. 2025. PMID: 39907648 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

