Insulin increases type I collagen synthesis in hepatic stellate cells via α5β1 integrin
- PMID: 40808720
- PMCID: PMC12345635
- DOI: 10.20517/mtod.2024.59
Insulin increases type I collagen synthesis in hepatic stellate cells via α5β1 integrin
Abstract
Aim: A direct effect of insulin on the synthesis of extracellular matrix proteins has been described in extrahepatic organs. The current study investigates the role of insulin in type I collagen production in hepatic stellate cells (HSCs).
Methods: Primary HSC cultures from wild-type mice and from L-SACC1 transgenic mice that exhibit hyperinsulinemia and resultant insulin resistance due to a defect in hepatic insulin clearance were used.
Results: Insulin significantly increased type I collagen synthesis in HSC primary cultures in the presence of high but not low glucose concentrations. Although HSCs contain a functional, insulin-activated PI3 kinase signaling pathway, insulin increases type I collagen synthesis by mechanisms independent of PI3 kinase. Insulin stimulated α5β1 integrin levels and phosphorylation of focal adhesion kinase, a major signaling mediator in the integrin pathway. In addition, α5β1 integrin siRNA interference abolished insulin-mediated type I collagen synthesis by HSCs. L-SACC1 mice showed increased hepatic collagen deposition as compared to wild-type mice. HSCs isolated from L-SACC1 mice synthesize more type I collagen and α5β1 integrin than HSCs isolated from wild-type controls.
Conclusion: Insulin exerts a direct profibrotic impact on HSCs by an α5β1 integrin-mediated mechanism, independently of the PI3 kinase signaling pathway. Thus, chronic hyperinsulinemia may potentiate liver collagen deposition in insulin resistance states. This likely increases the risk of significant fibrosis burden in chronic liver disease associated with insulin resistance.
Keywords: Collagen; hepatic stellate cells; insulin; insulin resistance; α5β1 integrin.
Conflict of interest statement
Conflicts of interest Najjar SM and Kasumov T are Editorial Board members of the journal Metabolism and Target Organ Damage. Najjar SM and Kasumov T were not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling and decision making. The other authors declare that there are no conflicts of interest.
Figures




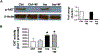


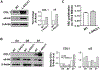

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Tudor staphylococcal nuclease (Tudor-SN) regulates activation of quiescent hepatic stellate cells.FEBS J. 2025 Jul;292(13):3545-3564. doi: 10.1111/febs.70073. Epub 2025 Mar 17. FEBS J. 2025. PMID: 40098321
-
MFAP4 Deficiency Attenuates Liver Fibrosis by Regulating Hepatic Stellate Cell Fate Through Inhibition of the FAK/PI3K/NFκB Signaling Pathway.Cell Mol Gastroenterol Hepatol. 2025 May 29;19(10):101548. doi: 10.1016/j.jcmgh.2025.101548. Online ahead of print. Cell Mol Gastroenterol Hepatol. 2025. PMID: 40449846 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes: a systematic review and economic modelling.Health Technol Assess. 2024 Dec;28(80):1-190. doi: 10.3310/JYPL3536. Health Technol Assess. 2024. PMID: 39673446 Free PMC article.
References
-
- El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460–8. - PubMed
-
- Bugianesi E, Marchesini G, Gentilcore E, et al. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: role of insulin resistance and hepatic steatosis. Hepatology 2006;44:1648–55. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
