Postmarketing Research for Opioid Abuse-Deterrent Formulations: A Narrative Review
- PMID: 40808815
- PMCID: PMC12345969
- DOI: 10.2147/JPR.S519421
Postmarketing Research for Opioid Abuse-Deterrent Formulations: A Narrative Review
Abstract
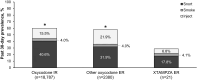
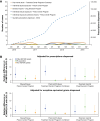
Although prescription opioids may be necessary to manage severe and persistent pain, many factors including concern for opioid abuse and misuse have led to restricted availability of these analgesics. Opioid abuse-deterrent formulations (ADFs) were developed to enhance resistance to tampering yet retain analgesic efficacy. US Food and Drug Administration (FDA) approval for the ADF designation is based on prespecified preclinical (category 1), pharmacokinetic (category 2), and/or clinical (category 3) evidence demonstrating abuse-deterrent properties. Currently, 4 opioid formulations carry the ADF designation: XTAMPZA® ER (oxycodone), OXYCONTIN® (oxycodone hydrochloride), HYSINGLA™ ER (hydrocodone bitartrate), and ROXYBOND™ (oxycodone hydrochloride). The FDA requires that ADFs undergo postapproval evaluation to assess their impact on meaningful reductions in abuse, misuse, and related clinical outcomes. An additional designation is available based on FDA assessment of these postmarket studies (category 4). However, none of the 4 opioid ADFs have yet attained this additional category 4 labeling. The impact of opioid ADFs on abuse, misuse, and related clinical outcomes is unclear. The objectives of this narrative review are 1) to describe the benefits of and need for ADFs; 2) to provide an overview of the FDA guidance for ADFs, with a focus on category 4 postmarketing requirements; and 3) to summarize select postmarketing studies of the ADF prescription opioids currently available in the US. We identified key postmarketing publications for these ADFs via PubMed searches and investigation of literature cited in relevant publications. Three opioid ADFs (XTAMPZA ER, OXYCONTIN, and HYSINGLA ER) currently report postmarketing research, generally demonstrating reduced nonoral abuse or misuse compared with non-ADFs or other ADFs. Of note, XTAMPZA ER has shown sustained lower levels of nonoral abuse or misuse compared with other ADFs, despite a substantial increase in dispensed prescriptions since its launch in 2016. Additional postmarketing research is needed, especially for HYSINGLA ER and ROXYBOND.
Keywords: ADF categories; hydrocodone; oxycodone; postmarketing research.
© 2025 Webster et al.
Conflict of interest statement
L.W.: Consultant for CognifiSense, Collegium, Elysium Pharmaceuticals, Ensysce Biosciences, Quivive Pharma, Salix Pharmaceuticals, Trevi Therapeutics. Advisory board participation for AdhereRx, Ensysce Biosciences, KemPharm, MedLogix. Travel expenses: AdhereRx, Elysium Pharmaceuticals, Ensysce Biosciences, PainScript. L.W. also reports personal fees for providing independent and evidence-based medical opinions, outside the submitted work. J.G.: Consultant for Acertis, Collegium, Ensysce, Hisamitsu, Kailo, Sanofi, Latigo, Xgene, and Tris. J.L.G.: Employee of Inflexxion, a division of Integrated Behavioral Health, which contracts with FDA and multiple sponsor companies with interests in some of the products included in the compounds evaluated for this article. J.L.G. is also a consultant for the Opioid Post-Marketing Requirement Consortium (OPC). C.E.A.: Grants and/or personal fees from AbbVie, Nevro Corp, Lilly, XGene Pharma, Lundbeck, Biohaven, Amgen, OPC, Collegium, Elsevier, Averitas, Scilex Holding, and Vertex. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Current Impact and Application of Abuse-Deterrent Opioid Formulations in Clinical Practice.Pain Physician. 2017 Nov;20(7):E1003-E1023. Pain Physician. 2017. PMID: 29149148
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2022 Jun 9;6(6):CD003870. doi: 10.1002/14651858.CD003870.pub7. Cochrane Database Syst Rev. 2022. PMID: 35679121 Free PMC article.
-
Opioids for cancer pain - an overview of Cochrane reviews.Cochrane Database Syst Rev. 2017 Jul 6;7(7):CD012592. doi: 10.1002/14651858.CD012592.pub2. Cochrane Database Syst Rev. 2017. PMID: 28683172 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

