An Advanced Healthcare Sensing Platform for Direct Detection of Viral Proteins in Seconds at Femtomolar Concentrations via Aerosol Jet 3D-Printed Nano and Biomaterials
- PMID: 40808895
- PMCID: PMC12347435
- DOI: 10.1002/admi.202400005
An Advanced Healthcare Sensing Platform for Direct Detection of Viral Proteins in Seconds at Femtomolar Concentrations via Aerosol Jet 3D-Printed Nano and Biomaterials
Abstract
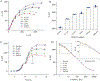
Sensing of viral antigens has become a critical tool in combating infectious diseases. Current sensing techniques have a tradeoff between sensitivity and time of detection; with 10-30 min of detection time at a relatively low sensitivity and 6-12 h of detection at a high (picomolar) sensitivity. In this research, uniquely nanoengineered interfaces are demonstrated on 3D electrodes that enable the detection of spike antigens of SARS-CoV-2 and their variants in seconds at femtomolar concentrations with excellent specificity, thus, overcoming this tradeoff. The 3D electrodes, manufactured using a high-resolution aerosol jet 3D nanoprinter, consist of a microelectrode array of sintered gold nanoparticles coated with graphene and antibodies specific to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike antigens. An impedance-based sensing modality is employed to sense several pseudoviruses of SARS-CoV-2 variants of concern (VOCs). This device is sensitive to most of the pseudoviruses of SARS-CoV-2 VOCs. A high sensitivity of 100 fm, along with a low limit-of-detection of 9.2 fm within a test range of 0.1-1000 pm, and a detection time of 43 s are shown. This work illustrates that effective nano-bioengineering of interfaces can be used to create an ultrafast and ultrasensitive healthcare diagnostic tool for combating emerging infections.
Keywords: 3D biosensor; nano-bio-interfaces; nanoprinting; pseudovirus; ultrarapid diagnosis; variants of concern.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Workplace interventions to reduce the risk of SARS-CoV-2 infection outside of healthcare settings.Cochrane Database Syst Rev. 2022 May 6;5(5):CD015112. doi: 10.1002/14651858.CD015112.pub2. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Apr 10;4:CD015112. doi: 10.1002/14651858.CD015112.pub3. PMID: 35514111 Free PMC article. Updated.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Electrophoresis.2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36251838 Free Books & Documents.
References
-
- Nathanson N, in Viral Pathogenesis, 3rd Ed., (Eds: Katze MG, Korth MJ, Law GL, Nathanson N), Academic Press, Boston: 2016.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
