A comprehensive comparison of third generation epidermal growth factor receptor tyrosine kinase inhibitors in the treatment efficacy and adverse events: A Bayesian meta-analysis
- PMID: 40808912
- PMCID: PMC12346681
- DOI: 10.1016/j.gmg.2025.100064
A comprehensive comparison of third generation epidermal growth factor receptor tyrosine kinase inhibitors in the treatment efficacy and adverse events: A Bayesian meta-analysis
Abstract
Background: It is a challenge for clinicians to choose the optimal third generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs) treatment for individual patients. In this meta-analysis we compare the efficacy of five third-generation EGFR-TKIs, as first-line and second-line therapies for non-small cell lung cancer (NSCLC) patients, and their adverse events (AEs).
Methods: A Bayesian hierarchical network meta-analysis was conducted to evaluate the hazard ratios (HR) of first-line therapeutic effects and AEs for these third-generation EGFR-TKIs comparing with first-generation EGFR-TKIs. Additionally, a simple comparison analysis was conducted to evaluate second-line therapeutic effects.
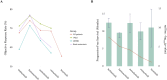
Results: All third-generation TKIs exhibited superior efficacy compared to Gefitinib in first-line treatment. Furmonertinib achieved the lowest HR in the exon 19 deletions subgroup (HR: 0.35; 95 % CI: 0.23-0.54), while Lazertinib showed the most favorable HR in the exon 21 L858R subgroup (HR: 0.44; 95 % CI: 0.28-0.70) and among patients with brain metastases (HR: 0.33; 95 % CI 0.18-0.59). In the second-line setting, Furmonertinib achieved the highest numerically objective response rate across the overall population (74.0 %; 95 % CI: 68.0-80.0 %) and all evaluated subgroups. Adverse event analysis showed that Furmonertinib had the lowest overall AE incidence, and Lazertinib had the lowest rate of high-grade (≥ grade 3) AEs.
Conclusions: All third-generation EGFR-TKIs exhibited favorable efficacy in both first- and second-line settings. Differences in AE profiles were also noted.
Keywords: Adverse events; EGFR mutations; Efficacy; Non-small cell lung cancer; Third-generation TKIs.
© 2025 International Knowledge Direct Medical Associations.
Conflict of interest statement
All authors have no relevant competing interests to report.
Figures



References
-
- Ferlay J., Ervik M., Lam F., Laversanne M., Colombet M., Mery L., et al. Global Cancer Observatory: Cancer today. Lyon, France: International Agency for Research on Cancer. Available from http://gco.iarc.who.int/today.accessed 2024–5-1. 2024.
-
- Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. - PubMed
-
- Shi Y., Chen G., Wang X., Liu Y., Wu L., Hao Y., et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir. Med. 2022;10(11):1019–1028. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

