Molecular subtypes, prognostic factors, and treatment optimization in pediatric medulloblastoma: a real-world study from China
- PMID: 40809009
- PMCID: PMC12343238
- DOI: 10.3389/fonc.2025.1597123
Molecular subtypes, prognostic factors, and treatment optimization in pediatric medulloblastoma: a real-world study from China
Abstract
Background: Medulloblastoma (MB) is the most common malignant pediatric brain tumor, yet systematic studies on molecular characteristics and treatment efficacy in Chinese pediatric MB remain scarce. This study evaluates recurrence and mortality risk factors and the feasibility of intensified chemotherapy.
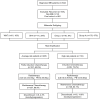
Methods: A retrospective analysis of 381 MB patients (WNT: 63, SHH: 106, Group 3: 27, Group 4: 185) was conducted. Kaplan-Meier analysis estimated survival rates, and Cox regression identified independent risk factors for recurrence and mortality.
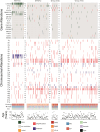
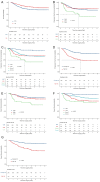
Results: With a median follow-up of 4.8 years, 5-year PFS and OS were 69.9% ± 2.4% and 80.6% ± 2.1%, respectively. WNT-MB had the best prognosis, while Group 3-MB had the worst. Independent recurrence risk factors included high-risk status (HR=2.931, p<0.001), TP53 mutation (HR=1.873, p<0.001), MYCN amplification (HR=1.52, p=0.001), chromosome 12p amplification, and 9q deletion. Mortality was associated with LC/A pathology (HR=1.573, p=0.007), TP53 mutation (HR=2.049, p<0.001), and high-risk status (HR=3.966, p<0.001). TP53 mutations influenced WNT-MB recurrence, and Group 3-MB showed a high recurrence risk even without MYC amplification or metastasis. No treatment-related fatalities were observed.
Conclusion: This study identified key clinical and molecular risk factors associated with recurrence and mortality in pediatric medulloblastoma. The findings underscore the prognostic relevance of TP53 mutations, MYCN amplification, and specific chromosomal alterations, particularly in non-metastatic subgroups. These insights may help guide risk-adapted and personalized treatment strategies in future studies.
Keywords: large cohort; maintain chemotherapy by risk stratification; medulloblastoma; real-world study; risk factors for relapse and mortality.
Copyright © 2025 Liu, Sun, Li, Du, Zhang, Zhang, Sun and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Molecular and clinical heterogeneity within MYC-family amplified medulloblastoma is associated with survival outcomes: A multicenter cohort study.Neuro Oncol. 2025 Jan 12;27(1):222-236. doi: 10.1093/neuonc/noae178. Neuro Oncol. 2025. PMID: 39377358 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
-
Impact of first-line chemoimmunotherapy with or without radiotherapy on the prognosis of patients with locally advanced or metastatic esophageal squamous cell carcinoma: a multicenter, real-world, retrospective cohort study from China (NCT06478355).Front Immunol. 2025 Jul 28;16:1633930. doi: 10.3389/fimmu.2025.1633930. eCollection 2025. Front Immunol. 2025. PMID: 40791599 Free PMC article. Clinical Trial.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Chemotherapy for children with medulloblastoma.Cochrane Database Syst Rev. 2015 Jan 1;1(1):CD006678. doi: 10.1002/14651858.CD006678.pub2. Cochrane Database Syst Rev. 2015. PMID: 25879092 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

