Copper metabolism and cuproptosis: broad perspectives in the treatment of hepatocellular carcinoma
- PMID: 40809021
- PMCID: PMC12343259
- DOI: 10.3389/fonc.2025.1555858
Copper metabolism and cuproptosis: broad perspectives in the treatment of hepatocellular carcinoma
Abstract
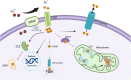
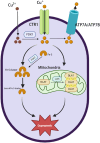
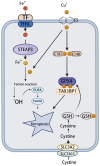
Copper is an essential trace element that plays a pivotal role in multiple biological processes, including energy production and angiogenesis. It is also a vital cofactor necessary for the maintenance of biological functions and has been implicated in cancer development. The recently identified form of cell death, cuproptosis, has a unique induction mechanism-accumulated copper ions directly bind to lipoylated proteins in the mitochondrial tricarboxylic acid (TCA) cycle, triggering toxic protein aggregation and cell death. This process can be specifically induced by oxidative stress and mitochondrial dysfunction, providing a novel direction for the development of anti-tumor strategies that target copper metabolism. In hepatocellular carcinoma (HCC), there is a significant correlation between disturbances in copper metabolism and abnormalities in the cuproptosis pathway. HCC cells maintain pro-carcinogenic copper levels through the upregulation of copper transporter proteins such as copper transporter 1 (CTR1). Conversely, the dysregulation of the expression of key genes involved in cuproptosis (ferredoxin 1, lipoic acid synthetase) may mediate treatment resistance. In this review, we focus on the mechanism by which cuproptosis influences the occurrence and development of HCC, evaluate its potential as a diagnostic biomarker, and examine therapeutic strategies targeting this form of cell death (nanocarrier-based delivery of copper ion carriers, CRISPR-mediated editing of copper-regulated genes). These strategies may provide a novel perspective for overcoming the current therapeutic limitations of HCC.
Keywords: copper; copper chelators; copper ionophores; cuproptosis; cuproptosis-related genes; hepatocellular carcinoma.
Copyright © 2025 Liang, Wang, Wu, Huang, Zhang and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cuproptosis: a novel therapeutic mechanism in lung cancer.Cancer Cell Int. 2025 Jun 24;25(1):231. doi: 10.1186/s12935-025-03864-1. Cancer Cell Int. 2025. PMID: 40555995 Free PMC article. Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Cuproptosis as the new kryptonite of cancer: a copper-dependent novel cell death mechanism with promising implications for the treatment of hepatocellular carcinoma.J Cancer Res Clin Oncol. 2023 Dec;149(19):17663-17670. doi: 10.1007/s00432-023-05456-w. Epub 2023 Oct 16. J Cancer Res Clin Oncol. 2023. PMID: 37843555 Free PMC article. Review.
-
Nanobubble-mediated sonodynamic therapy enhances cuproptosis in the treatment of hepatocellular carcinoma.Nanoscale Adv. 2025 Jun 12;7(15):4651-4659. doi: 10.1039/d5na00280j. eCollection 2025 Jul 22. Nanoscale Adv. 2025. PMID: 40556860 Free PMC article.
-
Cuproptosis: Current insights into its multifaceted role in disease, cancer, and translational/therapeutic opportunities.Biomed Pharmacother. 2025 Sep;190:118422. doi: 10.1016/j.biopha.2025.118422. Epub 2025 Aug 6. Biomed Pharmacother. 2025. PMID: 40774019 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

