Radioresistant mouse pheochromocytoma cell lines
- PMID: 40809026
- PMCID: PMC12344521
- DOI: 10.3389/fonc.2025.1517132
Radioresistant mouse pheochromocytoma cell lines
Abstract
Objective: Patients diagnosed with metastatic pheochromocytoma/paraganglioma (PCC/PGL) have limited treatment options. In some cases, peptide receptor radionuclide therapy (PRRT) is followed by an eruption of metastases, possibly originating from tumor cells with a radioresistant phenotype. However, the underlying mechanisms of radioresistance in PCC/PGL remain largely unknown and appropriate models are missing.
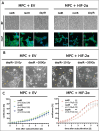
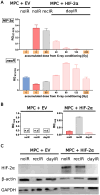
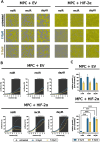
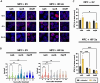
Methods: Two genetically modified mouse pheochromocytoma (MPC) cell lines, one positive and one negative for hypoxia-inducible factor 2α expression (MPC+HIF-2α and MPC+EV [empty vector], respectively), were X-ray-conditioned through fractionated irradiation at sublethal doses. Two procedures were tested: one allowed for recovery between each irradiation step (recIR), while the other demanded daily irradiation (dayIR). Changes in cell morphology, growth rates, and DNA repair (γH2AX immunostaining) were characterized in response to irradiation.
Results: We generated two MPC+HIF-2α- and two MPC+EV-derived cell lines that tolerate irradiations with X-rays at dose fractions of 2 Gy per day without significant growth inhibition. All recIR-and dayIR-conditioned cell lines showed increased DNA repair capacity. Morphological changes toward stronger clustering and slower growth were more pronounced in dayIR-conditioned than in recIR-conditioned cell lines. X-ray-conditioned MPC+HIF-2α cells showed the highest increase in resistance to X-ray-treatment with dose fractions up to 5 Gy per day.
Conclusion: The herein established X-ray-conditioned MPC cell lines represent PCC/PGL models with a radioresistant phenotype. Further investigations on the radiation-induced genetic responses of these cell lines, their corresponding tumor spheroids, and tumor allografts in mice will help to elucidate the underlying mechanisms of acquired radioresistance and radionuclide therapy-induced metastatic eruption in PCC/PGL. Lastly, the suitability of advanced PRRT and complementary treatments can be tested to improve theranostic strategies.
Keywords: HIF-2α; X-ray; irradiation; paraganglioma; pheochromocytoma; pseudohypoxia; radioresistance.
Copyright © 2025 Lemm, Gebhardt, Groß, Richter, Ullrich and Pietzsch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author JP declares that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Hereditary Paraganglioma-Pheochromocytoma Syndromes.2008 May 21 [updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2008 May 21 [updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301715 Free Books & Documents. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Efficacy and safety of allogeneic mesenchymal precursor cells with and without hyaluronic acid for treatment of chronic low back pain: a prospective, randomized, double blind, concurrent-controlled 36-month study.Spine J. 2025 Sep;25(9):1997-2013. doi: 10.1016/j.spinee.2025.03.015. Epub 2025 Mar 31. Spine J. 2025. PMID: 40174800
-
[Experimental study on promotion of skin radiation damage repair by icarin via HIF-2α/VEGF/Notch pathway to enhance the paracrine function of adipose-derived stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025 Jul 15;39(7):881-890. doi: 10.7507/1002-1892.202503089. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025. PMID: 40659593 Free PMC article. Chinese.
References
-
- Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype–phenotype correlations in pheochromocytoma and paraganglioma: a systematic review and individual patient meta-analysis(2019). Available online at: https://erc.bioscientifica.com/view/journals/erc/26/5/ERC-19-0024.xml (Accessed June 10, 2025)., PMID: - PMC - PubMed
LinkOut - more resources
Full Text Sources

