Synthetic niclosamide-loaded controlled-release nanospheres with high solubility and stability exerting multiple effects against Clostridioides difficile
- PMID: 40809058
- PMCID: PMC12343512
- DOI: 10.3389/fmicb.2025.1617631
Synthetic niclosamide-loaded controlled-release nanospheres with high solubility and stability exerting multiple effects against Clostridioides difficile
Abstract
Introduction: Niclosamide (NIC) has significant potential as a clinical therapeutic agent for Clostridioides difficile infection (CDI); however, its strong hydrophobicity hampers its oral bioavailability, and its active effects against C. difficile remain unclear.
Methods: Niclosamide-loaded controlled-release hyaluronic acid-modified poly (lactic-co-glycolic acid) naosphernes (NIC@PLGA-HAs) were synthesized using an oil-in-water emulsion technique and their effects on C. difficile cell growth, spore germination, biofilm formation, and NIC interaction sites with C. difficile toxin B (TcdB) were analyzed.
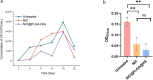
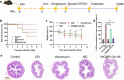
Results: NIC@PLGA-HAs exhibited enhanced solubility and stability, with a water contact angle on a hydrophilic surface of 65.1° and a zeta potential of 31.57 ± 2.08 mV, and pH-responsive (pH 7.4) controlled-release characteristics compared to free NIC. The NIC@PLGA-HAs killed C. difficile vegetative cells at a minimum inhibitory concentration (MIC) of 4 μg/mL. When C. difficile cells were treated with NIC@PLGA-HAs at the 1/4 MIC, spore germination and biofilm formation were significantly inhibited compared to those in untreated cells (P < 0.01). NIC was found to interact with the receptor-binding domain of TcdB at 24 amino acid sites via an enthalpy-driven reaction (enthalpy change, 36.21 kJ/mol and entropy change, 212.9 J⋅mol/K). In vivo experimental findings in Mongolian gerbils indicated that NIC@PLGA-HAs outperformed free NIC in reducing pathological damage, diarrhea severity, weight loss, and TcdB production and enhanced the survival rate.
Conclusion: These findings presented the therapeutic potential of NIC@PLGA-HAs with high solubility and stability, which simultaneously exerted multiple biological activities against C. difficile.
Keywords: Clostridioides difficile; Niclosamide; biofilm formation; loaded controlled-release nanospheres; multiple effects; spore germination.
Copyright © 2025 Tai, Zhang, Han, Hu, Lin, Zhai, Tian, Song, Wan, Chen and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Real-world use of bezlotoxumab to prevent recurrent Clostridioides difficile infections: a single-center experience and meta-analysis.Therap Adv Gastroenterol. 2025 Jun 21;18:17562848251346593. doi: 10.1177/17562848251346593. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40547251 Free PMC article.
-
Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children.Cochrane Database Syst Rev. 2017 Dec 19;12(12):CD006095. doi: 10.1002/14651858.CD006095.pub4. Cochrane Database Syst Rev. 2017. PMID: 29257353 Free PMC article.
-
Niclosamide attenuates erectile dysfunction and corporal fibrosis via reversal of Smad signaling in diabetic rat model.J Sex Med. 2024 Dec 1;21(12):1111-1119. doi: 10.1093/jsxmed/qdae129. J Sex Med. 2024. PMID: 39403936
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
LinkOut - more resources
Full Text Sources

