Real-world outcomes with ibrutinib in relapsed or refractory mantle cell lymphoma: a Danish population-based study
- PMID: 40809191
- PMCID: PMC12346063
- DOI: 10.1016/j.bneo.2025.100128
Real-world outcomes with ibrutinib in relapsed or refractory mantle cell lymphoma: a Danish population-based study
Abstract
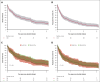
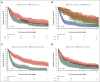
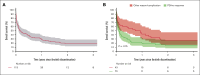
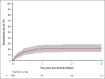
Ibrutinib was approved for relapsed/refractory (R/R) mantle cell lymphoma (MCL) based on high response rates in clinical trials, but it is unclear how effective ibrutinib is in the real-world setting. This study provides population-based response rates and survival estimates and characterization of prognostic indicators and adverse events (AEs) to ibrutinib for patients with R/R MCL. All patients diagnosed with MCL in Denmark from 2010 to 2022 were identified in the Danish Lymphoma Registry and screened for eligibility. Data were collected from health records. Patients receiving ibrutinib in second or later lines were included and followed from ibrutinib start until death or last follow-up. End points were overall response rate (ORR), progression-free survival (PFS), overall survival (OS), frequency of AEs, and AE-related discontinuation and dose reductions. In total, 146 patients were included (median age, 73 years); 90 (62%) received ibrutinib in second line. ORR was 56%, median PFS 5.8 months, and median OS 12.0 months. In Cox regressions, factors associated with inferior PFS were Ki67 of ≥50% (hazard ratio [HR], 2.34; 95% confidence interval [CI], 1.47-3.71), blastoid or pleomorphic subtype (HR, 3.00; 95% CI, 2.04-4.41), early relapses (HR, 1.65; 95% CI, 1.15-2.36), and refractory disease (HR, 1.57; 95% CI, 1.07-2.30). Three-year cumulative incidences of discontinuation and dose reductions owing to AEs were 19% and 22%, respectively. Median OS after ibrutinib discontinuation was 1.9 months. In conclusion, real-world outcomes after initiation of ibrutinib for R/R MCL were poorer than observed in clinical trials, and dose-limiting toxicities were common, emphasizing the need for more effective treatments and dose-optimization studies.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: T.T. received research support from Janssen as funding paid to the institution; and travel support from Immedica Pharma. A.L.A.-M. received research funding from Genentech. P.B. was on the advisory board of Roche, AbbVie, Bristol Myers Squibb, and SERB. T.S.L. received research support from Genentech; was on the advisory board of Roche, Bristol Myers Squibb, and Gilead; and received travel support from Gilead and Roche. M.R.C. was on the advisory board of AbbVie, AstraZeneca, Genmab, Gilead, Incyte, Janssen, and Roche; and received travel support from AbbVie, AstraZeneca, Genmab, Janssen, Pfizer, and Roche. M. Jerkeman received research support from 10.13039/100004337Roche, 10.13039/100006483AbbVie, 10.13039/100004325AstraZeneca, and 10.13039/100002491Bristol Myers Squibb; and honoraria from Kite/10.13039/100005564Gilead, 10.13039/100004337Roche, AbbVie, Janssen, 10.13039/100004325AstraZeneca, and 10.13039/100002491Bristol Myers Squibb. K.G. received research support from Janssen as funding paid to the institution; and was a consultant for 10.13039/100019120Otsuka Pharma and GlaxoSmithKline. The remaining authors declare no competing financial interests.
Figures

Similar articles
-
Efficacy and safety of ibrutinib in mantle cell lymphoma: A systematic review and meta-analysis.Daru. 2022 Dec;30(2):367-378. doi: 10.1007/s40199-022-00444-w. Epub 2022 Sep 3. Daru. 2022. PMID: 36057010 Free PMC article.
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Indirect Treatment Comparisons of Ibrutinib Versus Physician's Choice and Idelalisib Plus Ofatumumab in Patients With Previously Treated Chronic Lymphocytic Leukemia.Clin Ther. 2017 Jan;39(1):178-189.e5. doi: 10.1016/j.clinthera.2016.12.001. Epub 2017 Jan 3. Clin Ther. 2017. PMID: 28062113
-
Ibrutinib plus venetoclax in relapsed or refractory mantle cell lymphoma (SYMPATICO): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study.Lancet Oncol. 2025 Feb;26(2):200-213. doi: 10.1016/S1470-2045(24)00682-X. Lancet Oncol. 2025. PMID: 39914418 Clinical Trial.
References
-
- Glimelius I, Smedby KE, Eloranta S, Jerkeman M, Weibull CE. Comorbidities and sex differences in causes of death among mantle cell lymphoma patients – a nationwide population-based cohort study. Br J Haematol. 2020;189(1):106–116. - PubMed
-
- Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175(3):410–418. - PubMed
LinkOut - more resources
Full Text Sources

