Prophylactic azithromycin for chronic lung allograft dysfunction following lung transplantation: a systematic review and meta-analysis
- PMID: 40809223
- PMCID: PMC12340312
- DOI: 10.21037/jtd-2025-365
Prophylactic azithromycin for chronic lung allograft dysfunction following lung transplantation: a systematic review and meta-analysis
Abstract
Background: Azithromycin (AZI) has proven effective in improving pulmonary function and survival in certain patients with established chronic lung allograft dysfunction (CLAD) following lung transplantation (LTx), but its prophylactic effects on CLAD remain controversial. This study aimed to assess the outcomes of prophylactic AZI for CLAD following LTx.
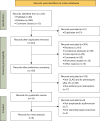
Methods: A systematic review was conducted based on PubMed, Embase and Cochrane Library. All included studies reported the primary or secondary outcomes in the prophylactic azithromycin (pAZI) and control groups. The CLAD onset, disease-free survival and overall survival (OS) data were pooled using fixed-effect or random-effect models. Sensitivity analysis was employed to evaluate the robustness of the pooled results, while a funnel plot was utilized to assess the publication bias.
Results: Six eligible studies involving 1,251 LTx recipients were included. The pooled analysis revealed a lower risk of CLAD onset in the pAZI group compared to the control group [relative risk (RR) 0.64, 95% confidence interval (CI): 0.51-0.81, P<0.001]. Moreover, the pAZI group exhibited superiority in the 3-year [hazard ratio (HR) 0.57, 95% CI: 0.39-0.83, P=0.003] and 5-year CLAD-free survival (HR 0.61, 95% CI: 0.43-0.86, P=0.005); but this superiority was not observed in the 3-year (HR 0.69, 95% CI: 0.31-1.54, P=0.36) and 5-year OS (HR 0.59, 95% CI: 0.30-1.14, P=0.12).
Conclusions: Prophylactic AZI may reduce the risk of CLAD onset and improve 3- and 5-year CLAD-free survival, providing supporting evidence for its application in LTx community. More high-quality and well-designed studies are warranted to determine the prophylactic effects of AZI on CLAD and its phenotypes following LTx.
Keywords: Azithromycin (AZI); chronic lung allograft dysfunction (CLAD); lung transplantation (LTx); prophylaxis; treatment.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-365/coif). The authors have no conflicts of interest to declare.
Figures






Similar articles
-
Microbiome and metabolome patterns after lung transplantation reflect underlying disease and chronic lung allograft dysfunction.Microbiome. 2024 Oct 9;12(1):196. doi: 10.1186/s40168-024-01893-y. Microbiome. 2024. PMID: 39385282 Free PMC article.
-
Bisphosphonates in multiple myeloma: an updated network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 18;12(12):CD003188. doi: 10.1002/14651858.CD003188.pub4. Cochrane Database Syst Rev. 2017. PMID: 29253322 Free PMC article.
-
Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2013 Nov 28;(11):CD009764. doi: 10.1002/14651858.CD009764.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Oct 30;10:CD009764. doi: 10.1002/14651858.CD009764.pub3. PMID: 24288145 Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Sex and gender as predictors for allograft and patient-relevant outcomes after kidney transplantation.Cochrane Database Syst Rev. 2024 Dec 19;12(12):CD014966. doi: 10.1002/14651858.CD014966.pub2. Cochrane Database Syst Rev. 2024. PMID: 39698949
References
-
- Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021;40:1060-72. 10.1016/j.healun.2021.07.021 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
