Real-time magnetic resonance imaging guided accelerator in stereotactic body radiation therapy for non-small cell lung cancer
- PMID: 40809238
- PMCID: PMC12340310
- DOI: 10.21037/jtd-24-686
Real-time magnetic resonance imaging guided accelerator in stereotactic body radiation therapy for non-small cell lung cancer
Abstract
Background: Stereotactic body radiotherapy (SBRT) is crucial for lung tumor treatment, but facing challenges like intra-fractional anatomical changes and organ risks. Magnetic resonance-guided online adaptive SBRT (MRg-SBRT) is an innovative technique promising safer delivery of ablative doses and protect organ at risk (OAR). This study aimed to investigate the feasibility of the whole process of MRg-SBRT for non-small cell lung cancer (NSCLC) patients.
Methods: Physical parameters of radiotherapy for 23 cases of NSCLC who underwent MRg-SBRT were retrospectively analyzed. This included patients' treatment course, planned target volume (PTV), target area coverage, and OAR recipient volume. The focus was on inter- and intra-fraction MR real-time monitoring to correct off-target. Local control and adverse event outcomes of patients' SBRT treatment were also retrospectively analyzed.
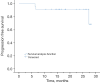
Results: All 23 patients completed treatment on time without any treatment interruptions or pauses due to the MR. Tumor movement was predominantly in the superior-inferior (SI) directions through Elekta Unity real-time online monitoring. The baseline plan was altered in four patients, and an adaptive plan was used to correct inter- and intra-fraction off-targeting in a timely manner. The prescribed dose coverage for PTV was 95.3%, with a median bilateral lung volume 20 (V20) GY of 6.3%, and a maximal dose to the spinal cord of 117.3 cGy. The response results showed that the disease control rate (DCR) was 100% with an objective response rate (ORR) of 82.6%. Follow-up results showed an acute grade one to two pneumonia incidence of 82.6% and grade three pneumonias in one patient.
Conclusions: MRg-SBRT can guide treatment plans for the clinical needs of SBRT for lung cancer patients, especially for old patients, proving the feasibility of SBRT for lung lesions with MRg-SBRT, and online real-time monitoring reduces intra- and inter-fraction off-targeting, and guarantees the treatment efficiency of patients without significantly increasing the incidence of serious adverse events.
Keywords: Magnetic resonance-guided radiotherapy (MR-guided radiotherapy); non-small cell lung cancer (NSCLC); stereotactic body radiotherapy (SBRT).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-686/coif). The authors have no conflicts of interest to declare.
Figures
Similar articles
-
CT-based online adaptive radiotherapy improves target coverage and organ at risk (OAR) avoidance in stereotactic body radiation therapy (SBRT) for prostate cancer.Clin Transl Radiat Oncol. 2023 Oct 24;44:100693. doi: 10.1016/j.ctro.2023.100693. eCollection 2024 Jan. Clin Transl Radiat Oncol. 2023. PMID: 38021093 Free PMC article.
-
Biological adaptive radiotherapy for short-time dose compensation in lung SBRT patients.Med Phys. 2025 Jul;52(7):e17820. doi: 10.1002/mp.17820. Epub 2025 Apr 14. Med Phys. 2025. PMID: 40229143
-
Ablative Five-Fraction CT Versus MR-Guided Stereotactic Body Radiation Therapy for Pancreatic Cancer: In Silico Evaluation of Interfraction Anatomic Changes as a Rationale for Online Adaptive Replanning.Cancers (Basel). 2025 Jun 20;17(13):2061. doi: 10.3390/cancers17132061. Cancers (Basel). 2025. PMID: 40647362 Free PMC article.
-
Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery.Cochrane Database Syst Rev. 2018 Jul 9;7(7):CD011492. doi: 10.1002/14651858.CD011492.pub2. Cochrane Database Syst Rev. 2018. PMID: 29987845 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
LinkOut - more resources
Full Text Sources