Combining recombinant human endostatin with third-generation EGFR-TKIs in advanced EGFR-sensitive mutant non-small cell lung cancer
- PMID: 40809253
- PMCID: PMC12340283
- DOI: 10.21037/jtd-2025-1223
Combining recombinant human endostatin with third-generation EGFR-TKIs in advanced EGFR-sensitive mutant non-small cell lung cancer
Abstract
Background: Third-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are standard first-line options in advanced or metastatic EGFR mutant non-small cell lung cancer (NSCLC). This study aimed to compare the efficacy and safety of third generation EGFR-TKIs combined with recombinant human endostatin (Endostar) versus EGFR-TKIs alone in previously untreated advanced epidermal growth factor receptor (EGFR) mutant NSCLC patients.
Methods: A total of 118 untreated advanced EGFR-sensitive-mutant NSCLC patients from a single center were retrospectively included in the study. Of the patients, 71 received third-generation EGFR-TKIs (the T group) and 47 received combination of Endostar and third-generation EGFR-TKIs therapy (the E + T group). Progression-free survival (PFS), overall survival (OS), the objective response rate (ORR), the disease control rate (DCR), and adverse events (AEs) were evaluated.
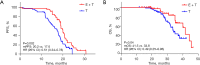
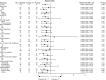
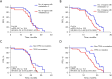
Results: Compared to the T group, the E + T group had a significantly higher ORR (91.5% vs. 77.5%; P=0.047), and improved PFS (20.2 vs. 17.6 months; P=0.002) and OS (41.5 vs. 33.8 months; P=0.04). However, there was no significant difference in the DCR between the two groups (97.9% vs. 97.2%; P>0.99). Multivariate analysis identified the Eastern Cooperative Oncology Group performance status (ECOG-PS) score, brain metastasis, EGFR co-mutation, and treatment regimen as independent prognostic factors. Subgroup analysis showed that the E + T group had greater clinical benefits for patients with ≥2 distant metastatic organs (P=0.01) and EGFR/TP53 co-mutations (P=0.01). The incidence of AEs of any level was higher in the E + T group than the T group (53.2% vs. 45.1%, P=0.39).
Conclusions: In this real-world study, the combination of recombinant human endostatin and third-generation EGFR-TKIs significantly improved the ORR, PFS, and OS in previously untreated advanced EGFR-mutant NSCLC patients and thus represents a promising treatment option that requires further prospective evaluation.
Keywords: Endostar; Non-small cell lung cancer (NSCLC); epidermal growth factor receptor mutation (EGFR mutation); epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-1223/coif). A.C.T. receives grants (to institution) from Takeda and AstraZeneca; honoraria from Roche, AstraZeneca, Guardant, Merck, Amgen, Takeda; participated in Advisory Board from Amgen, Bayer, Pfizer. The other authors have no conflicts of interest to declare.
Figures

Similar articles
-
Adjuvant epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) for the treatment of people with resected stage I to III non-small-cell lung cancer and EGFR mutation.Cochrane Database Syst Rev. 2025 May 27;5(5):CD015140. doi: 10.1002/14651858.CD015140.pub2. Cochrane Database Syst Rev. 2025. PMID: 40421698 Review.
-
Feasibility and safety of EGFR-TKI neoadjuvant therapy for EGFR-mutated NSCLC: A meta-analysis.Eur J Clin Pharmacol. 2024 Apr;80(4):505-517. doi: 10.1007/s00228-024-03620-w. Epub 2024 Feb 1. Eur J Clin Pharmacol. 2024. PMID: 38300281
-
Comparison of the efficacy and safety of first-line treatments based on clinicopathological characteristics for patients with advanced epidermal growth factor receptor mutated non-small-cell lung cancer: A systematic review and network meta-analysis.Crit Rev Oncol Hematol. 2022 Sep;177:103760. doi: 10.1016/j.critrevonc.2022.103760. Epub 2022 Jul 21. Crit Rev Oncol Hematol. 2022. PMID: 35870763
-
Epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation testing in adults with locally advanced or metastatic non-small cell lung cancer: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2014 May;18(32):1-166. doi: 10.3310/hta18320. Health Technol Assess. 2014. PMID: 24827857 Free PMC article.
-
Prediction of the efficacy and clinical prognosis of first-line EGFR-tyrosine kinase inhibitors in non-small cell lung cancer patients based on ΔCt values derived from the super-amplification refractory mutation system (ARMS): a real-world retrospective study.J Thorac Dis. 2025 Jun 30;17(6):3897-3911. doi: 10.21037/jtd-2025-97. Epub 2025 Jun 25. J Thorac Dis. 2025. PMID: 40688317 Free PMC article.
References
-
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. 10.1200/JCO.2010.33.4235 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
