Real-world efficacy of atezolizumab combined with etoposide and platinum chemotherapy in extensive-stage small cell lung cancer: a retrospective cohort study
- PMID: 40809273
- PMCID: PMC12340325
- DOI: 10.21037/jtd-2025-1049
Real-world efficacy of atezolizumab combined with etoposide and platinum chemotherapy in extensive-stage small cell lung cancer: a retrospective cohort study
Abstract
Background: Small cell lung cancer (SCLC) is a highly aggressive thoracic malignancy for which immune checkpoint inhibitors (ICIs) combined with chemotherapy have become standard first-line therapy according to recent randomized clinical trials. However, real-world data on the efficacy of atezolizumab-based regimens remain limited, especially in patients with high tumor burden or brain metastases. The aim of this study was to evaluate the real-world effectiveness of atezolizumab plus etoposide and platinum (EP) chemotherapy compared to EP alone as first-line treatment in patients with extensive-stage SCLC (ES-SCLC).
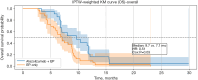
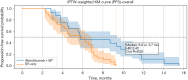
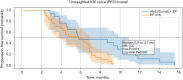
Methods: This retrospective cohort study included patients diagnosed with ES-SCLC at Tianjin Chest Hospital between January 2019 and December 2024. Eligible patients received either atezolizumab plus EP or EP chemotherapy alone as first-line treatment, with at least four completed cycles. Inverse probability of treatment weighting (IPTW) was used to balance the baseline covariates. The primary outcomes were overall survival (OS) and progression-free survival (PFS), which were analyzed with Kaplan-Meier curves and Cox proportional hazards models with robust variance estimation.
Results: A total of 95 patients were included (42 in the atezolizumab group and 53 in the EP-only group). After IPTW adjustment, the combination group demonstrated improved OS as compared to the EP-only group [median 9.7 vs. 7.1 months; hazard ratio (HR) =0.51, 95% confidence interval (CI): 0.30-0.94]; meanwhile, the PFS showed similar median values between the groups (5.8 vs. 5.7 months), but the immunotherapy group (atezolizumab plus etoposide and platinum) exhibited a delayed separation in survival curves and a favorable HR (0.42, 95% CI: 0.21-0.86), suggesting durable benefit. Subgroup analysis revealed significant OS and PFS benefits among patients without brain metastases, while results in the brain metastasis subgroup were inconclusive due to the limited sample size.
Conclusions: In this real-world cohort of patients with ES-SCLC, atezolizumab combined with EP chemotherapy was associated with improved survival outcomes as compared to EP alone, even in a clinically heterogeneous population. These findings support the broader applicability of immune-chemotherapy in routine practice and underscore the potential benefit for patients without brain metastases.
Keywords: Atezolizumab; extensive-stage small cell lung cancer (ES-SCLC); immune-chemotherapy; inverse probability of treatment weighting (IPTW); real-world study.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-1049/coif). The authors have no conflicts of interest to declare.
Figures





Similar articles
-
Impact of delayed addition of PD-1/PD-L1 inhibitors to chemotherapy on outcomes in patients with extensive-stage small cell lung cancer.Ther Adv Med Oncol. 2025 Jul 20;17:17588359251356919. doi: 10.1177/17588359251356919. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40697877 Free PMC article.
-
Toripalimab Plus Chemotherapy as a First-Line Therapy for Extensive-Stage Small Cell Lung Cancer: The Phase 3 EXTENTORCH Randomized Clinical Trial.JAMA Oncol. 2025 Jan 1;11(1):16-25. doi: 10.1001/jamaoncol.2024.5019. JAMA Oncol. 2025. PMID: 39541202 Clinical Trial.
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitors combined with or without anti-angiogenesis therapy for extensive-stage small-cell lung cancer: a network meta-analysis.Ther Adv Med Oncol. 2025 Jun 25;17:17588359251348310. doi: 10.1177/17588359251348310. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40574965 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
References
-
- Falchero L, Guisier F, Darrason M, et al. Long-term effectiveness and treatment sequences in patients with extensive stage small cell lung cancer receiving atezolizumab plus chemotherapy: Results of the IFCT-1905 CLINATEZO real-world study. Lung Cancer 2023;185:107379. 10.1016/j.lungcan.2023.107379 - DOI - PubMed
-
- National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 2.2022). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf - PMC - PubMed
LinkOut - more resources
Full Text Sources
