Ultrasonographic hip morphology in mucopolysaccharidosis type I Hurler after hematopoietic stem cell gene therapy
- PMID: 40809356
- PMCID: PMC12339489
- DOI: 10.1177/18632521251349438
Ultrasonographic hip morphology in mucopolysaccharidosis type I Hurler after hematopoietic stem cell gene therapy
Abstract
Purpose: To assess ultrasonographic features of hip morphology in mucopolysaccharidosis type I Hurler.
Methods: Acetabular bony rim, acetabular cartilaginous roof, alpha and beta angles, echogenicity, and hip coverage were analyzed in eight mucopolysaccharidosis type I Hurler syndrome children before and after hematopoietic stem cell gene therapy.
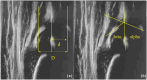
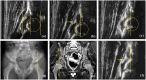
Results: Sixteen hips at baseline, 10 at +12 months, and 10 at + 24 months after hematopoietic stem cell gene therapy were evaluated. The median age was 22, 35, and 45 months at baseline evaluation, +12, and +24 months, respectively. Acetabular bony rim at baseline was angular in 2/16, rounded in 10/16, notched in 2/16, and flattened in 2/16; at +12 months, angular in 2/10, rounded in 5/10, notched in 3/10; at +24 months, angular in 2/10, rounded in 3/10, irregular in 1/10, and notched in 4/10. Acetabular cartilaginous roof at baseline was normal in 4/16, enlarged in 12/16; at +12 months, enlarged in 10/10 and at +24 months, enlarged in 8/10 and normal in 2/10. Echogenicity of the joint capsule at baseline was normal in 10/16, increased in 6/16; at +12 months, normal in 8/10, increased in 2/10; at +24 months, normal in 6/10, increased in 4/10. The mean femoral head coverage was 60% at baseline (16/16), 62% at +12 months (10/10), and 52% at +24 months (2/10). The mean alpha angle was 60° at baseline (16/16), 64° at +12 months (10/10), and 60° (2/10) at +24 months. The mean beta angle was 67° at baseline (16/16), 65° at +12 months (10/10), and 49° at +24 months (8/10).
Conclusions: Hip morphology of children with mucopolysaccharidosis type I Hurler syndrome before and after hematopoietic stem cell gene therapy can be evaluated by available ultrasound techniques until a median age of 45 months.
Keywords: Hip morphology; gene therapy; hip dysplasia; mucopolysaccharidosis; ultrasonography.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AA was the study PI between May 2018 and July 2020, and MEB has been the PI since July 2020. GC, MC, FT, CF, AA, and MEB are investigators of the trial. MEB has acted as ad hoc consultants for an Orchard Therapeutics advisory board in 2020. MEB has also received occasional reimbursement of travel costs and registration fees for scientific congress presentations from Orchard Therapeutics. MDP has a service contract with Telethon Foundation. The other authors declare that there is no conflict of interest.
Figures


References
-
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D. (eds) The Metabolic and Molecular Basis of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001, pp. 3421–52.
-
- Muenzer J, Wraith JE, Clarke LA, et al. Mucopoly-saccharidosis I: management and treatment guidelines. Pediatrics 2009; 123(1): 19–29. - PubMed
-
- Russell C, Hendson G, Jevon G, et al. Murine MPS I: insights into the pathogenesis of Hurler syndrome. Clin Genet 1998; 53(5): 349–361. - PubMed
-
- Thawrani DP, Walker K, Polgreen LE, et al. Hip dysplasia in patients with Hurler syndrome (mucopolysaccharidosis type 1H). J Pediatr Orthop 2013; 33(6): 635–643. - PubMed
LinkOut - more resources
Full Text Sources
