The dimethyloxalylglycine-functionalized nanofibers for in situ regeneration of infected developing dental roots
- PMID: 40809404
- PMCID: PMC12343479
- DOI: 10.1016/j.mtbio.2025.102062
The dimethyloxalylglycine-functionalized nanofibers for in situ regeneration of infected developing dental roots
Abstract
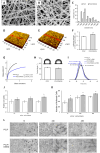
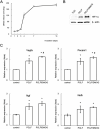
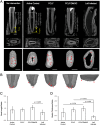
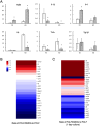
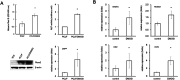
In situ regeneration in restorative dentistry targets the repair of tissues directly at the injury site by utilizing engineered biomaterials to guide endogenous cell activity. This approach aims to simplify treatment procedures and achieve more predictable outcomes, thus to supports the regeneration of damaged tissues and potentially restores tooth vitality, reducing the need for more invasive treatments. This study explores the potential of poly(ε-caprolactone) fibers (PCLF) functionalized with a hypoxia-inducible factor 1-alpha (HIF-1α) stabilizing small molecule dimethyloxalylglycine (DMOG) for in situ regeneration in the context of dental root repair in developing immature teeth. PCLF functionalized with DMOG (PCLF/DMOG) was applied to regenerative endodontic procedure (REP) treatment of infected developing dental roots, and its biologic properties and therapeutic potential were investigated through both in vitro studies and in vivo experiments, focusing on their capacity to promote in situ regeneration. In vivo application demonstrated the effectiveness of PCLF/DMOG in promoting root development, apical closure, and improving infectious lesions, contrasting with contemporary REP treatment controls that showed unpredictable outcomes. Mechanistically, the sustained release of DMOG from PCLF/DMOG significantly enhanced the expression of HIF-1α and upregulated expression of genes associated with angiogenesis and neurogenesis, including VEGF-α and NGF. The PCLF/DMOG upregulated antimicrobial peptides, facilitated efferocytic activities, promoted macrophage polarization to the M2 phenotype, and mobilized mesenchymal stem cells. Taken together, PCLF/DMOG could enhance innate immune responses and foster favorable microenvironment to guide cellular differentiation, promoting in situ regeneration of dental roots in the inflammatory microenvironments.
Keywords: HIF-1α; In situ regeneration; Infected immature dental roots; Macrophages; Mesenchymal stem cells; Nanofibrous scaffolds.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





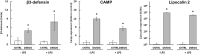
Similar articles
-
Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration-A Systematic Review.Int J Mol Sci. 2024 Mar 30;25(7):3879. doi: 10.3390/ijms25073879. Int J Mol Sci. 2024. PMID: 38612687 Free PMC article.
-
Cinobufagin Inhibits Invasion and Migration of Non-Small Cell Lung Cancer via Regulating Glucose Metabolism Reprogramming in Tumor-Associated Macrophages.Drug Des Devel Ther. 2025 Aug 2;19:6647-6664. doi: 10.2147/DDDT.S531190. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40771860 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Oncostatin-M functionalized cryogel microspheres for promoting diabetic bone defects regeneration.J Orthop Translat. 2025 Jun 20;53:138-148. doi: 10.1016/j.jot.2025.06.002. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40606844 Free PMC article.
-
Effectiveness of endodontic tissue engineering in treatment of apical periodontitis: A systematic review.Int Endod J. 2023 Oct;56 Suppl 3:533-548. doi: 10.1111/iej.13784. Epub 2022 Jun 23. Int Endod J. 2023. PMID: 35699668
References
-
- Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5(9):686–705. doi: 10.1038/s41578-020-0209-x. - DOI
-
- Lopushenko I., Sieryi O., Bykov A., Meglinski I. Exploring the evolution of circular polarized light backscattered from turbid tissue-like disperse medium utilizing generalized Monte Carlo modeling approach with a combined use of Jones and Stokes-Mueller formalisms. J. Biomed. Opt. 2024;29(5) doi: 10.1117/1.JBO.29.5.052913. - DOI - PMC - PubMed
-
- Siqueira J.F., Jr., Antunes H.S., Perez A.R., Alves F.R.F., Mdala I., Silva E., Belladonna F.G., Rocas I.N. The apical root canal system of teeth with posttreatment apical periodontitis: correlating microbiologic, tomographic, and histopathologic findings. J. Endod. 2020;46(9):1195–1203. doi: 10.1016/j.joen.2020.05.020. - DOI - PubMed
LinkOut - more resources
Full Text Sources

