Developing a procedure mimicking transvaginal mesh implantation in women in a modified POP rat model
- PMID: 40809433
- PMCID: PMC12343489
- DOI: 10.3389/fmed.2025.1603161
Developing a procedure mimicking transvaginal mesh implantation in women in a modified POP rat model
Abstract
Introduction: This study aims to establish a simple and reproducible transvaginal mesh surgery rat model based on the modified pelvic organ prolapse rat model.
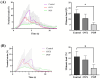
Methods: A total of 24 10-week-old female nulliparous Wistar rats were used in this study. The control group consisted of six rats with no interventions. The ovariectomy group included six rats that underwent bilateral ovariectomy. The pelvic organ prolapse group comprised 12 rats that underwent cervical pendant modeling 2 weeks after bilateral ovariectomy. Fourteen days post-modeling, six rats from the pelvic organ prolapse group underwent transvaginal mesh surgery. The rat pelvic organ prolapse quantification system was used to evaluate the prolapse condition of the rats before and after pelvic organ prolapse modeling, as well as after transvaginal mesh surgery. Vaginal wall tissue was collected to assess biomechanical changes before and after pelvic organ prolapse modeling. Additionally, vaginal wall and sacral ligament tissues were collected to evaluate structural changes and collagen alterations before and after pelvic organ prolapse modeling.
Results: The pelvic organ prolapse rat model exhibits anatomical prolapse, biomechanical changes, and pathological changes, including collagen fiber rupture and reduced collagen density. In contrast, the transvaginal mesh rat model demonstrates anatomical recovery in prolapsed rats.
Conclusion: This study successfully modified the pre-existing rat model of pelvic organ prolapse and effectively mimicked human transvaginal mesh surgery using this model.
Keywords: animal model; biomechanical; pelvic organ prolapse; rat; transvaginal mesh.
Copyright © 2025 Wang, Long, Yan, Li, Gao, Deqiong and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Surgical management of pelvic organ prolapse in women.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD004014. doi: 10.1002/14651858.CD004014.pub5. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Nov 30;11:CD004014. doi: 10.1002/14651858.CD004014.pub6. PMID: 23633316 Updated.
-
Transvaginal mesh or grafts or native tissue repair for vaginal prolapse.Cochrane Database Syst Rev. 2024 Mar 13;3(3):CD012079. doi: 10.1002/14651858.CD012079.pub2. Cochrane Database Syst Rev. 2024. PMID: 38477494 Free PMC article.
-
Surgical management of pelvic organ prolapse in women.Cochrane Database Syst Rev. 2010 Apr 14;(4):CD004014. doi: 10.1002/14651858.CD004014.pub4. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD004014. doi: 10.1002/14651858.CD004014.pub5. PMID: 20393938 Updated.
-
Perioperative interventions in pelvic organ prolapse surgery.Cochrane Database Syst Rev. 2018 Aug 19;8(8):CD013105. doi: 10.1002/14651858.CD013105. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2025 Jul 22;7:CD013105. doi: 10.1002/14651858.CD013105.pub2. PMID: 30121957 Free PMC article. Updated.
-
Perioperative interventions in pelvic organ prolapse surgery.Cochrane Database Syst Rev. 2025 Jul 22;7(7):CD013105. doi: 10.1002/14651858.CD013105.pub2. Cochrane Database Syst Rev. 2025. PMID: 40693510 Review.
References
-
- Darzi S, Alappadan J, Paul K, Mazdumder P, Rosamilia A, Truong YB, et al. Immunobiology of foreign body response to composite PLACL/gelatin electrospun nanofiber meshes with mesenchymal stem/stromal cells in a mouse model: Implications in pelvic floor tissue engineering and regeneration. Biomater Adv. (2023) 155:213669. 10.1016/j.bioadv.2023.213669 - DOI - PubMed
LinkOut - more resources
Full Text Sources

