Advances in cGAS-STING Signaling in Fibrosis Diseases: Therapeutic Target in Pathological Scars
- PMID: 40809466
- PMCID: PMC12345947
- DOI: 10.2147/JIR.S541656
Advances in cGAS-STING Signaling in Fibrosis Diseases: Therapeutic Target in Pathological Scars
Abstract
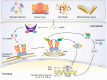
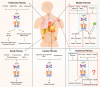
Fibrosis is characterised by an excessive response to tissue injury during wound healing, resulting in excessive scarring, which can affect any organ and lead to deformity or death. Fibrogenesis is a highly orchestrated process in which extracellular matrix deposition becomes unstructured, disrupting normal tissue architecture and subsequently impairing proper organ function through complex molecular signals and cellular responses. Inflammation is an important trigger for both regeneration and fibrosis after tissue damage-particularly due to inflammatory cytokines released by various recruited and activated immune cells-which can provoke an excessive inflammatory response in a short time. The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway has emerged as a key mediator of inflammation in the context of infection, cellular stress, tissue damage, and fibrosis. This reflects its capacity to sense and regulate cellular responses to ubiquitous danger-associated molecular patterns, mainly microbial or host-derived DNA. The cGAS-STING pathway plays a pivotal role in the development and progression of fibrotic diseases by linking cellular stress and DNA damage to chronic inflammation and fibroblast activation, thereby driving pathological tissue remodeling and extracellular matrix accumulation. However, a systematic summary of cGAS-STING in fibrotic diseases is lacking. Therefore, this review focuses on the effects and molecular mechanisms of cGAS-STING signalling in fibrotic diseases. We outline the principal elements of the cGAS-STING signalling cascade and discuss the mechanisms underlying the association of cGAS-STING activity with fibrosis in different organs. Finally, we elucidate the recently developed cGAS and STING antagonists and summarise their potential clinical applications in fibrotic diseases.
Keywords: cGAS-STING; extracellular matrix; fibroblast; fibrosis; inflammation.
© 2025 Zhao et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

