The development of an ingestible biosensor for the characterization of gut metabolites related to major depressive disorder: hypothesis and theory
- PMID: 40809492
- PMCID: PMC12342046
- DOI: 10.3389/fsysb.2023.1274184
The development of an ingestible biosensor for the characterization of gut metabolites related to major depressive disorder: hypothesis and theory
Abstract
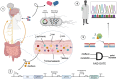
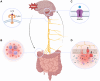
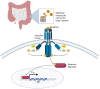
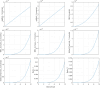
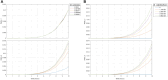
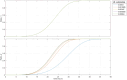
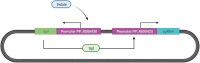
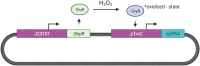
The diagnostic process for psychiatric conditions is guided by the Diagnostic and Statistical Manual of Mental Disorders (DSM) in North America. Revisions of the DSM over the years have led to lowered diagnostic thresholds across the board, incurring increased rates of both misdiagnosis and over-diagnosis. Coupled with stigma, this ambiguity and lack of consistency exacerbates the challenges that clinicians and scientists face in the clinical assessment and research of mood disorders such as Major Depressive Disorder (MDD). While current efforts to characterize MDD have largely focused on qualitative approaches, the broad variations in physiological traits, such as those found in the gut, suggest the immense potential of using biomarkers to provide a quantitative and objective assessment. Here, we propose the development of a probiotic Escherichia coli (E. coli) multi-input ingestible biosensor for the characterization of key gut metabolites implicated in MDD. DNA writing with CRISPR based editors allows for the molecular recording of signals while riboflavin detection acts as a means to establish temporal and spatial specificity for the large intestine. We test the feasibility of this approach through kinetic modeling of the system which demonstrates targeted sensing and robust recording of metabolites within the large intestine in a time- and dose- dependent manner. Additionally, a post-hoc normalization model successfully controlled for confounding factors such as individual variation in riboflavin concentrations, producing a linear relationship between actual and predicted metabolite concentrations. We also highlight indole, butyrate, tetrahydrofolate, hydrogen peroxide, and tetrathionate as key gut metabolites that have the potential to direct our proposed biosensor specifically for MDD. Ultimately, our proposed biosensor has the potential to allow for a greater understanding of disease pathophysiology, assessment, and treatment response for many mood disorders.
Keywords: gene editing; gut brain axis; gut microbiome; ingestible biosensor; major depressive disorder; personalized healthcare.
Copyright © 2023 Densil, George, Mahdi, Chami, Mark, Luo, Wang, Ali, Tang, Dong, Pao, Suri, Valentini, Al-Arabi, Liu, Singh, Wu, Peng, Sudharshan, Naqvi, Hewitt, Andary, Leung, Forsythe and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Omega-3 fatty acids for depression in adults.Cochrane Database Syst Rev. 2015 Nov 5;2015(11):CD004692. doi: 10.1002/14651858.CD004692.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Nov 24;11:CD004692. doi: 10.1002/14651858.CD004692.pub5. PMID: 26537796 Free PMC article. Updated.
-
Omega-3 fatty acids for depression in adults.Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD004692. doi: 10.1002/14651858.CD004692.pub5. Cochrane Database Syst Rev. 2021. PMID: 34817851 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Akoglu B., Schrott M., Bolouri H., Jaffari A., Kutschera E., Caspary W. F., et al. (2008). The folic acid metabolite L-5-methyltetrahydrofolate effectively reduces total serum homocysteine level in orthotopic liver transplant recipients: a double-blind placebo-controlled study. Eur. J. Clin. Nutr. 62, 796–801. 10.1038/SJ.EJCN.1602778 - DOI - PubMed
-
- Almario C. V., Megana L., Ballal W. D. C., Nordstrom C., Khanna D., Spiegel B. M. R. (2018). Burden of gastrointestinal symptoms in the United States: results of a nationally representative survey of over 71,000 Americans. Am. J. Gastroenterology 113 (11), 1701–1710. 10.1038/s41395-018-0256-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

