Timed photothermal therapy combining fluorescence-on chemotherapy maximizes tumor treatment
- PMID: 40809510
- PMCID: PMC12344197
- DOI: 10.1016/j.bioactmat.2025.07.051
Timed photothermal therapy combining fluorescence-on chemotherapy maximizes tumor treatment
Abstract
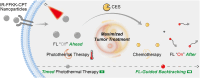
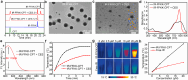
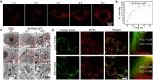
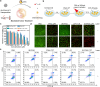
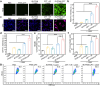
Photothermal therapy (PTT) combined with chemotherapy is a promising strategy for tumor treatment. However, its efficacy is often limited by the uncertainty of the irradiation timing of the photothermal agent. Herein, we rationally designed a self-assembling peptide-drug conjugate, IR775-Phe-Phe-Lys(CPT)-Lys(Biotin)-OH (IR-FFKK-CPT), which spontaneously self-assembles into fluorescence (FL)-quenched nanoparticles with high photothermal conversion efficiency. After being uptaken by the cancer cells, the nanoparticles are hydrolysed by carboxylesterase and disassembled to release CPT, turning the FL "On". The "On" FL displays not only the initiation of chemotherapy but also the decline of PTT efficacy. By leveraging the "On" FL as a temporal indicator, we precisely backtrack the optimal cell/tumor irradiation timing to be 4 h/12 h post-incubation/injection in cells/tumors. Subsequent therapeutic studies demonstrated that the timed irradiation on tumor at 12 h post injection significantly maximized tumor treatment outcomes, with average relative tumor volume on day 14 reduced to 13.7 % or 10.2 % of that in the groups of 6 h or 24 h, respectively. Guided by this timed PTT, IR-FFKK-CPT achieved an excellent tumor growth inhibition rate of 96.2 %, significantly outperforming the four positive control groups which showed tumor inhibition rates of 26.3 %-34.1 %. Our self-regulating theranostic strategy, that synchronizes timed PTT with visualized chemotherapy to maximize tumor treatment, provides people with a promising approach for precise tumor therapy.
Keywords: Chemotherapy; Disassembly; Irradiation timing; Nanoparticle; Photothermal therapy.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Arafeh R., Shibue T., Dempster J.M., Hahn W.C., Vazquez F. The present and future of the cancer dependency map. Nat. Rev. Cancer. 2025;25(1):59–73. - PubMed
-
- Tannock I.F. Conventional cancer therapy: promise broken or promise delayed? Lancet. 1998;351:SII9–SII16. - PubMed
-
- Wyld L., Audisio R.A., Poston G.J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 2015;12(2):115–124. - PubMed
-
- De Ruysscher D., Niedermann G., Burnet N.G., Siva S., Lee A.W.M., Hegi-Johnson F. Radiotherapy toxicity. Nat. Rev. Dis. Primers. 2019;5(1):13. - PubMed
-
- Chabner B.A., Roberts T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5(1):65–72. - PubMed
LinkOut - more resources
Full Text Sources

