Spore-inspired inhalation drug delivery system for asthma therapy
- PMID: 40809511
- PMCID: PMC12347991
- DOI: 10.1016/j.bioactmat.2025.07.045
Spore-inspired inhalation drug delivery system for asthma therapy
Abstract
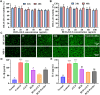
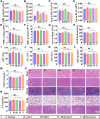
The delivery efficiency of drugs in the lung is crucial for inhaled therapies targeting pulmonary diseases. However, current inhalation carriers face challenges overcoming pulmonary barriers, leading to insufficient delivery efficiency. To tackle this limitation, we have developed a "spore-inspired" strategy. Ganoderma lucidum spores (GLS) provide dual delivery advantages: their natural morphology promotes bronchial-alveolar deposition while evading macrophage endocytosis, enhancing pulmonary retention. Using these features, a biomimetic carrier called carbonized GLS (cGLS) is created through precise carbonization, which preserves the spores' natural morphological benefits while reducing the immune response and increasing drug-loading capacity. Subsequently, we develop the spore-inspired inhalation drug delivery system BUD-cGLS by loading the asthma medication budesonide (BUD), which facilitates accurate regulation of the "deposition-escape-release" process. In the OVA-induced asthma model, BUD-cGLS significantly reduces airway resistance, suppresses mucin secretion, and decreases inflammatory cytokines. Overall, these findings highlight the potential of this spore-inspired carrier as a promising inhalation platform for delivering drugs to treat asthma and other pulmonary diseases.
Keywords: Asthma; Budesonide; Dry powder inhaler; Pulmonary delivery; Spore-inspired carrier.
© 2025 The Authors.
Conflict of interest statement
The authors declare that no competing interest exists.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.Health Technol Assess. 2008 May;12(19):iii-iv, 1-360. doi: 10.3310/hta12190. Health Technol Assess. 2008. PMID: 18485271
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children.Cochrane Database Syst Rev. 2013 Apr 30;2013(4):CD007313. doi: 10.1002/14651858.CD007313.pub3. Cochrane Database Syst Rev. 2013. PMID: 23633340 Free PMC article.
References
-
- Han M.K., Hanania N.A., Martinez F.J. Confronting the challenge of copd: what is new in the approaches to diagnosis, treatment, and patient outcomes. Chest. 2018;154:984–985. - PubMed
-
- Safiri S., Carson-Chahhoud K., Karamzad N., Sullman M.J.M., Nejadghaderi S.A., Taghizadieh A., Bell A.W., Kolahi A.A., Ansarin K., Mansournia M.A., Collins G.S., Kaufman J.S. Prevalence, deaths, and disability-adjusted life-years due to asthma and its attributable risk factors in 204 countries and territories, 1990-2019. Chest. 2022;161:318–329. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

