Real-World Efficacy of Upadacitinib in Patients With Ulcerative Colitis
- PMID: 40809638
- PMCID: PMC12347216
- DOI: 10.7759/cureus.87903
Real-World Efficacy of Upadacitinib in Patients With Ulcerative Colitis
Abstract
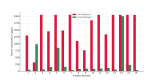
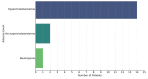
Upadacitinib (UPA) is a selective JAK-1 inhibitor used in the treatment of ulcerative colitis (UC) and Crohn's disease. We report a real-world retrospective study evaluating the efficacy and safety of UPA in 17 patients who scaled from mild to severe UC, and demonstrated significant improvement in Inflammatory biomarkers, with the majority (16/17 patients) achieving reduced fecal calprotectin levels. Three patients had subsequent endoscopic evaluation after being initiated on UPA; two showed improvement in the Mayo score, and the remaining patient remained static. The side effect profile was consistent with known JAK inhibitor effects, with elevated cholesterol as the most common adverse event, and one case of neutropenia was observed. These findings suggest that UPA is an effective therapeutic option for refractory UC patients, and further studies would help in confirming its long-term efficacy in these patients.
Keywords: crohn's disease; fecal calprotectin; inflammatory bowel disease; jak inhibitor; mayo score; ulcerative colitis; upadacitinib.
Copyright © 2025, Ghaffar et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: Dr. Fraz Ahmad and Dr. Linta Naveed have a personal relationship. This has been disclosed in accordance with ICMJE guidelines and institutional policies. The relationship has not influenced the design, analysis, interpretation, or authorship of this manuscript.
Figures




References
-
- Upadacitinib for Crohn's disease and ulcerative colitis treatment: hitting the selective JAKpot. Parigi TL, D'Amico F, Danese S. https://www.gastrojournal.org/article/S0016-5085(20)30517-5/fulltext. Gastroenterology. 2021;160:1472–1474. - PubMed
-
- Entering the era of disease modification in inflammatory bowel disease. Hart AL, Rubin DT. https://www.gastrojournal.org/article/S0016-5085(22)00141-X/fulltext. Gastroenterology. 2022;162:1367–1369. - PubMed
-
- In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) Parmentier JM, Voss J, Graff C, et al. https://link.springer.com/article/10.1186/s41927-018-0031-x. BMC Rheumatol. 2018;2:23. - PMC - PubMed
-
- Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn's disease. Sandborn WJ, Feagan BG, Loftus EV Jr, et al. https://www.sciencedirect.com/science/article/pii/S0016508520301670. Gastroenterology. 2020;158:2123–2138. - PubMed
-
- Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Sandborn WJ, Ghosh S, Panes J, et al. https://www.sciencedirect.com/science/article/pii/S0016508520302419. Gastroenterology. 2020;158:2139–2149. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
