Heavy metals trigger distinct molecular transformations in microplastic-versus natural-derived dissolved organic matter
- PMID: 40809685
- PMCID: PMC12346021
- DOI: 10.1016/j.ese.2025.100610
Heavy metals trigger distinct molecular transformations in microplastic-versus natural-derived dissolved organic matter
Abstract
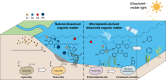
Dissolved organic matter (DOM) is a key determinant of heavy metal fate in aquatic environments, influencing their mobility, toxicity, and bioavailability. Derived from natural sources such as soil and vegetation decomposition, natural DOM (N-DOM) typically features humic-like substances with abundant oxygen-containing functional groups that stabilize heavy metals through complexation. However, microplastic-derived DOM (MP-DOM), increasingly prevalent due to plastic degradation, may interact differently with heavy metals, potentially exacerbating environmental risks amid rising plastic pollution. Yet, how heavy metals drive molecular transformations in MP-DOM versus N-DOM remains unclear, hindering accurate pollution assessments. Here, we compare interactions between N-DOM and MP-DOM with cadmium, chromium (Cr), copper, and lead from both fluorescence and molecular perspectives. Our results show that N-DOM, dominated by humic-like substances (46.0-57.3 %), lignin-like (55.0-64.9 %), and tannin-like (10.1-17.6 %) compounds, forms more stable heavy metal complexes via carboxyl, phenolic hydroxyl, and ether groups than MP-DOM. By contrast, MP-DOM-enriched in protein/phenolic-like substances (13.8-24.0 %), condensed aromatic (12.1-28.5 %), and protein/aliphatic-like (8.6-12.4 %) compounds-yields less stable complexes and is highly susceptible to Cr-induced oxidation. Mass-difference network analysis and density functional theory calculations further reveal that both DOM types undergo heavy-metal-triggered decarboxylation and dealkylation, but N-DOM retains complex structures, whereas MP-DOM degrades into smaller, hazardous molecules such as phenol and benzene. This study underscores the potential for heavy metals to exacerbate the ecological risks associated with the transformation of MP-DOM, providing crucial insights to inform global risk assessment and management strategies in contaminated waters where plastic and metal pollution co-occur.
Keywords: Complexation; Dissolved organic matter; Heavy metal; Microplastic; Molecular transformation.
© 2025 Published by Elsevier B.V. on behalf of Chinese Society for Environmental Sciences, Harbin Institute of Technology, Chinese Research Academy of Environmental Sciences.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Molecular-Scale Insights into the Surface Structural Transformation and Light-Driven Production of Reactive Oxygen Species of Goethite Induced by Microplastic-Derived Dissolved Organic Matter.Environ Sci Technol. 2025 Aug 6. doi: 10.1021/acs.est.5c07871. Online ahead of print. Environ Sci Technol. 2025. PMID: 40770887
-
Tracking microplastic-derived dissolved organic matter in the adsorption of its mixtures with natural organic matter via end-member mixing analysis.Environ Res. 2025 Jun 13;283:122144. doi: 10.1016/j.envres.2025.122144. Online ahead of print. Environ Res. 2025. PMID: 40517929
-
AI-assisted systematic review on remediation of contaminated soils with PAHs and heavy metals.J Hazard Mater. 2024 Apr 15;468:133813. doi: 10.1016/j.jhazmat.2024.133813. Epub 2024 Feb 17. J Hazard Mater. 2024. PMID: 38402679
-
Heavy metals: toxicity and human health effects.Arch Toxicol. 2025 Jan;99(1):153-209. doi: 10.1007/s00204-024-03903-2. Epub 2024 Nov 20. Arch Toxicol. 2025. PMID: 39567405 Free PMC article. Review.
References
-
- Fang S., Fang Z., Hua C., Zhu M., Tian Y., Yong X., Yang J., Ren L. Distribution, sources, and risk analysis of heavy metals in sediments of xiaoqing river basin, Shandong Province, China. Environ. Sci. Pollut. Control Ser. 2023;30(52):112445–112461. - PubMed
-
- Shaaban M., Nunez-Delgado A. Soil adsorption potential: harnessing earth's living skin for mitigating climate change and greenhouse gas dynamics. Environ. Res. 2024;251 - PubMed
-
- Olatunji O.S., Osibanjo O. North Central Nigeria; 2013. Eco-Partitioning and Indices of Heavey Metal Accumulation in Sediment and Tilapia zillii Fish in Water Catchment of River Niger at Ajaokuta.
-
- Hemachandra S.C.S.M., Sewwandi B.G.N. Application of water pollution and heavy metal pollution indices to evaluate the water quality in st. Sebastian canal, Colombo, Sri Lanka. Environ. Nanotechnol. Monit. Manag. 2023;20
-
- Weihua T., Lei W., Jianling G., Shuting W., Yu Z. Heavy metal pollution and source analysis of weihe river in Shaanxi Province. J. Environ. Eng. Technol. 2017;7(6):684–690.
LinkOut - more resources
Full Text Sources

