Exosomal miR-2137 from cadmium-treated hepatocytes drives renal ferroptosis via GPX4 suppression and is alleviated by selenium
- PMID: 40809692
- PMCID: PMC12344268
- DOI: 10.3389/fcell.2025.1585106
Exosomal miR-2137 from cadmium-treated hepatocytes drives renal ferroptosis via GPX4 suppression and is alleviated by selenium
Abstract
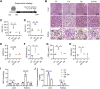
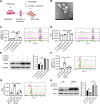
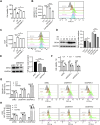
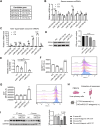
Cadmium (Cd) is a toxic heavy metal that primarily affects the liver and kidneys. Despite greater Cd accumulation in the liver, Cd-induced oxidative damage is more pronounced in the kidney, suggesting the involvement of hepatorenal communication. However, the underlying mechanism remains unclear. To investigate Cd-induced hepatorenal toxicity, we established a Cd-exposed mouse model and assessed ferroptosis-related liver and kidney injury. Exosomes derived from Cd-exposed hepatocytes were isolated, and miRNAs targeting GPX4 were screened and identified. The role of GPX4-targeting miRNAs in mediating renal toxicity induced by hepatocyte-derived exosomes was evaluated in vivo using antagomirs. The protective effect of selenium (Se) supplementation against Cd-induced hepatic and renal damage was also examined. Cd exposure induced significant liver and kidney injury through GPX4-downregulated ferroptosis. Mechanistically, exosomes from Cd-treated hepatocytes were enriched in miR-2137, which targets renal GPX4 and promotes ferroptosis in the kidney. Injection of hepatocyte-derived exosomes alone reduced renal GPX4 levels in vivo, an effect that was reversed by miR-2137 antagomir treatment. Furthermore, Se supplementation restored GPX4 expression and protected both liver and kidney tissues from Cd-induced damage. These findings reveal a novel exosome-mediated hepatorenal communication pathway under Cd exposure, wherein hepatocyte-derived exosomal miRNAs contribute to distant renal injury. Targeting specific exosomal miRNAs or enhancing GPX4 expression via selenium may offer therapeutic strategies against Cd toxicity.
Keywords: GPx4; cadmium; ferroptosis; hepatorenal communication; selenium.
Copyright © 2025 Wen, Qi, Wu, Ji and Zhan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

