A scoping review and evidence map of radiofrequency field exposure and genotoxicity: assessing in vivo, in vitro, and epidemiological data
- PMID: 40809778
- PMCID: PMC12343714
- DOI: 10.3389/fpubh.2025.1613353
A scoping review and evidence map of radiofrequency field exposure and genotoxicity: assessing in vivo, in vitro, and epidemiological data
Abstract
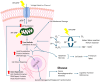
Background: Studies investigating genotoxic effects of radiofrequency electromagnetic field (RF-EMF) exposure (3 kHz-300 GHz) have used a wide variety of parameters, and results have been inconsistent. A systematic mapping of existing research is necessary to identify emerging patterns and to inform future research and policy.
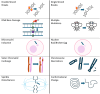
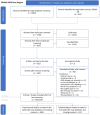
Methods: Evidence mapping was conducted using guidance from the Preferred Reporting Items for Systematic reviews and Meta-Analyses for Scoping Reviews (PRISMA-ScR). A comprehensive search strategy was applied across multiple research databases, using specific inclusion and exclusion criteria within each knowledge domain. Quantitative aggregation using tables, graphs and heat maps was used to synthesize data according to study type, organism type, exposure level and duration, biological markers (genotoxicity, cellular stress, apoptosis), RF-EMF signal characteristics, as well as funding source to further contextualize the evidence landscape. Quality criteria were applied as part of a focused analysis to explore potential biases and their effects on outcomes.
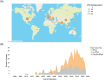
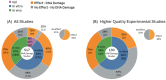
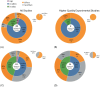
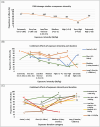
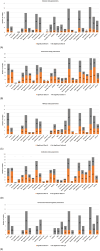
Results: Over 500 pertinent studies were identified, categorized as in vitro (53%), in vivo (37%), and epidemiological (10%), and grouped according to type of DNA damage, organism, intensity, duration, signal characteristics, biological markers and funding source. In vitro studies predominantly showed proportionally fewer significant effects, while in vivo and epidemiological studies showed more. DNA base damage studies showed the highest proportion of effects, as did studies using GSM talk-mode, pulsed signals and real-world devices. A complex relationship was identified between exposure intensity and duration, with duration emerging as a critical determinant of outcomes. A complex U-shaped dose-response relationship was evident, suggesting adaptive cellular responses, with increased free radical production as a plausible mechanism. Higher-quality studies showed fewer significant effects; however, the funding source had a stronger influence on outcomes than study quality. Over half (58%) of studies observing DNA damage used exposures below the International Commission of Non-Ionizing Radiation Protection (ICNIRP) limits.
Conclusion: The collective evidence reveals that RF-EMF exposures may be genotoxic and could pose a cancer risk. Exposure duration and real-world signals are the most important factors influencing genotoxicity, warranting further focused research. To address potential genotoxic risks, these findings support the adoption of precautionary measures alongside existing thermal-based exposure guidelines.
Keywords: apoptosis; cancer; electromagnetic radiation; genotoxicity; oxidative stress; radio frequencies; wireless technology.
Copyright © 2025 Weller, McCredden, Leach, Chu and Lam.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
The effects of radiofrequency electromagnetic fields exposure on tinnitus, migraine and non-specific symptoms in the general and working population: A systematic review and meta-analysis on human observational studies.Environ Int. 2024 Jan;183:108338. doi: 10.1016/j.envint.2023.108338. Epub 2023 Dec 6. Environ Int. 2024. PMID: 38104437
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
References
-
- Stewart BW, Kleihues P. World Cancer Report 2003. Lyon: IARCPress; (2003).
-
- Boyle P, Levin B. World Cancer Report. Lyon: International Agency for Research on Cancer; (2008).
-
- Stewart B, Wild C. Wolrd Cancer Report. Geneva: WHO Press; (2014).
-
- Wild CP, Weiderpass E, Stewart BW (editors). World Cancer Report: Cancer Research for Cancer Prevention. Lyon: International Agency for Research on Cancer; (2020). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

