Multifaceted Human Antigen R (HuR): A Key Player in Liver Metabolism and MASLD
- PMID: 40809813
- PMCID: PMC12341429
- DOI: 10.3390/livers5030033
Multifaceted Human Antigen R (HuR): A Key Player in Liver Metabolism and MASLD
Abstract
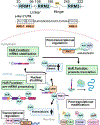
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the leading cause of chronic liver disease worldwide, affecting approximately 25-30% of the global adult population and highlighting the urgent need for effective therapeutics and prevention strategies. MASLD is characterized by excessive hepatic lipid accumulation and can progress, in a subset of patients, to metabolic dysfunction-associated steatohepatitis (MASH), a pro-inflammatory and pro-fibrotic condition associated with increased risk of liver cirrhosis and hepatocellular carcinoma. Although the molecular drivers of MASLD progression remain incompletely understood, several key metabolic pathways-such as triglyceride handling, cholesterol catabolism, bile acid metabolism, mitochondrial function, and autophagy-are consistently dysregulated in MASLD livers. This narrative review summarizes primary literature and highlights insights from recent reviews on the multifaceted role of the mRNA-binding protein Human antigen R (HuR) in the post-transcriptional regulation of critical cellular processes, including nutrient metabolism, cell survival, and stress responses. Emerging evidence underscores HuR's essential role in maintaining liver homeostasis, particularly under metabolic stress conditions characteristic of MASLD, with hepatocyte-specific HuR depletion associated with exacerbated disease severity. Moreover, comorbid conditions such as obesity, type 2 diabetes mellitus, and cardiovascular disease not only exacerbate MASLD progression but also involve HuR dysregulation in extrahepatic tissues, further contributing to liver dysfunction. A deeper understanding of HuR-regulated post-transcriptional networks across metabolic organs may enable the development of targeted therapies aimed at halting or reversing MASLD progression.
Keywords: RNA binding proteins; hepatic steatosis; human antigen R (HuR); metabolic dysfunction-associated steatotic liver disease (MASLD); metabolic syndrome; non-alcoholic fatty liver disease (NAFLD); post-transcriptional regulation.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflicts of interest.
Figures

Similar articles
-
Metabolic Dysfunction-Associated Steatotic Liver Disease (MΑSLD).2025 Aug 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Aug 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31082077 Free Books & Documents.
-
Metabolic dysfunction-associated steatotic liver disease: A story of muscle and mass.World J Gastroenterol. 2025 May 28;31(20):105346. doi: 10.3748/wjg.v31.i20.105346. World J Gastroenterol. 2025. PMID: 40495947 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Metabolic-dysfunction associated steatotic liver disease and atrial fibrillation: A review of pathogenesis.World J Cardiol. 2025 Jun 26;17(6):106147. doi: 10.4330/wjc.v17.i6.106147. World J Cardiol. 2025. PMID: 40575425 Free PMC article. Review.
-
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in People With Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association.Diabetes Care. 2025 Jul 1;48(7):1057-1082. doi: 10.2337/dci24-0094. Diabetes Care. 2025. PMID: 40434108 Review.
References
-
- Wong VWS; Ratziu V; Bugianesi E; Francque S; Zelber-Sagi S; Valenti L; Roden M; Schick F; Yki-Järvinen H; Gastaldelli A; et al. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. - PubMed
-
- Huang DQ; Wong VWS; Rinella ME; Boursier J; Lazarus JV; Yki-Järvinen H; Loomba R Metabolic dysfunction-associated steatotic liver disease in adults. Nat. Rev. Dis. Prim. 2025, 11, 14. - PubMed
-
- Wallace C; Gamkrelidze I; Estes C; Razavi H; Sanyal AJ Modeling the health and economic impact of pharmacologic therapies for MASLD in the United States. J. Hepatol. 2025, 83, 21–30. - PubMed
-
- Miao L; Targher G; Byrne CD; Cao Y-Y; Zheng M-H Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
