Year-Round Analysis of Multiphase Sulfate Production in Aerosol Particles in East Asia
- PMID: 40809908
- PMCID: PMC12340764
- DOI: 10.1021/acsestair.5c00136
Year-Round Analysis of Multiphase Sulfate Production in Aerosol Particles in East Asia
Abstract
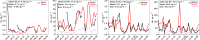
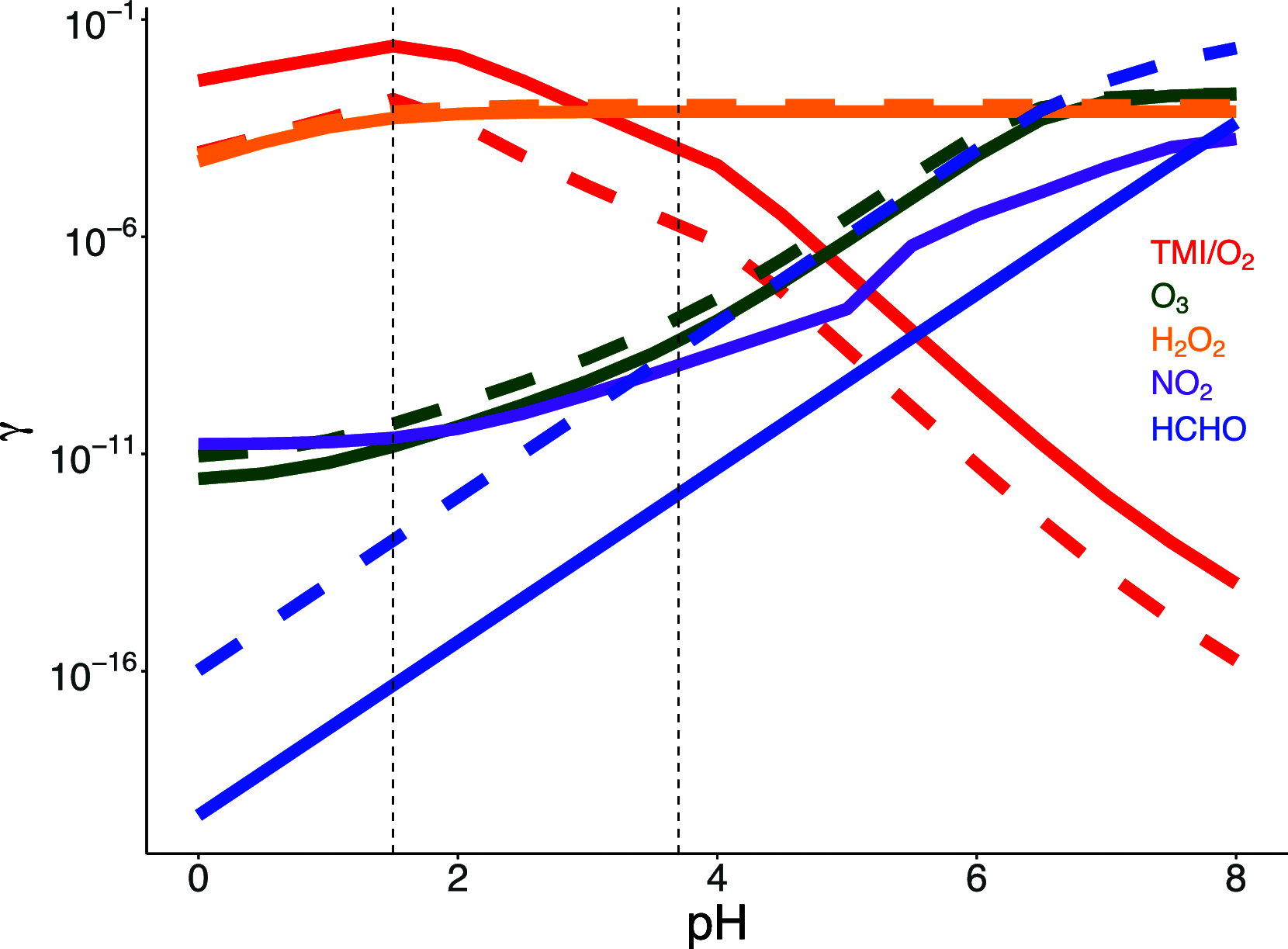
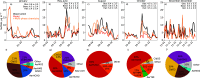
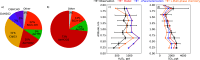
Missing sulfate production pathways have been implicated as the cause of model underestimates of sulfate during haze events in East Asia. We add multiphase oxidation of SO2 in aerosol particles by H2O2, O3, NO2, HCHO, and O2, catalyzed by transition metal ions (TMIs), to the GEOS-Chem model and evaluate the model with (1) year-round ground-based observations in Seoul, South Korea, (2) airborne observations from the KORUS-AQ field campaign, and (3) fall and winter ground-based observations in Beijing, China. Multiphase chemistry contributes 14% to 90% to total sulfate production depending on the location and season and increases model daily average sulfate by 2 to 3 μg m-3, with maximum daily increases up to 12 μg m-3. From winter to summer, oxidation pathways shift, with the largest fraction of multiphase sulfate production occurring during spring and summer due to oxidation by H2O2. Multiphase oxidation of SO2 by the H2O2 pathway reduces gas-phase H2O2 concentrations by -40% in spring, which improves model agreement with H2O2 airborne observations. Oxidation pathways also shift between cities, in particular the contribution from the TMI and NO2 pathways, which are more important in Beijing than in Seoul. This is due to higher levels of transition metals, and a larger impact of an overly shallow mixed layer in Beijing compared to Seoul. The implementation of multiphase aerosol chemistry in GEOS-Chem here allows for the use of this chemistry in other models that can address boundary layer errors, including WRF-GC and CESM-GC. The analysis presented here shows that this chemistry is important to the simulation of sulfate year-round, not only during haze events, and is unique in showing coupled gas- and aerosol-phase impacts of multiphase chemistry.
Keywords: KORUS-AQ; aerosol; east Asia; haze; model; sulfate.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Modeling attainment in Fairbanks, Alaska, for the wintertime PM2.5 24-hour non-attainment area using the CMAQ (community multi-scale air quality) model.Faraday Discuss. 2025 Jun 16;258(0):234-264. doi: 10.1039/d4fd00158c. Faraday Discuss. 2025. PMID: 40042620
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Wang Y., Zhang Q., Jiang J., Zhou W., Wang B., He K., Duan F., Zhang Q., Philip S., Xie Y.. Enhanced Sulfate Formation during China’s Severe Winter Haze Episode in January 2013 Missing from Current Models. J. Geophys. Res.:Atmos. 2014;119(17):10,425–10,440. doi: 10.1002/2013JD021426. - DOI
-
- Zheng B., Zhang Q., Zhang Y., He K. B., Wang K., Zheng G. J., Duan F. K., Ma Y. L., Kimoto T.. Heterogeneous Chemistry: A Mechanism Missing in Current Models to Explain Secondary Inorganic Aerosol Formation during the January 2013 Haze Episode in North China. Atmos. Chem. Phys. 2015;15(4):2031–2049. doi: 10.5194/acp-15-2031-2015. - DOI
-
- Gao M., Carmichael G. R., Wang Y., Ji D., Liu Z., Wang Z.. Improving Simulations of Sulfate Aerosols during Winter Haze over Northern China: The Impacts of Heterogeneous Oxidation by NO2. Front. Environ. Sci. Eng. 2016;10(5):16. doi: 10.1007/s11783-016-0878-2. - DOI
LinkOut - more resources
Miscellaneous
