Positioning and sequencing of advanced therapies in inflammatory bowel disease: A guide for clinical practice
- PMID: 40809925
- PMCID: PMC12344364
- DOI: 10.3748/wjg.v31.i29.107745
Positioning and sequencing of advanced therapies in inflammatory bowel disease: A guide for clinical practice
Abstract
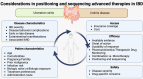
Over the past decade, the therapeutic armamentarium for inflammatory bowel disease (IBD) has substantially expanded with the incorporation of multiple classes of advanced therapies. Currently, in addition to tumor necrosis factor-α inhibitors, the therapeutic arsenal for IBD includes anti-integrin agents, interleukin (IL)-12/23p40 and IL-23p19 antibodies, Janus kinase inhibitors, and sphingosine 1-phosphate receptor modulators. Although advances in IBD pharmacotherapy have enabled disease remission and improved control of intestinal inflammation in many individuals previously considered clinically 'intractable', they have also increased the complexity of decision-making related to the initial positioning and sequencing of therapies in the heterogeneous clinical presentations of IBD. Until molecular and genetic markers capable of predicting therapeutic responses become available in practice, the choice of initial and subsequent therapy in individuals with IBD is based on factors including disease severity, phenotype, risk of complications, comorbidities, extraintestinal manifestations, and the balance between efficacy, safety, convenience, and access. This review explores the factors that influence treatment decisions regarding initial therapy selection and sequencing across IBD scenarios, offering practical tips for personalizing therapy based on the safety and efficacy of advanced treatments and the individual's risk of disease- or therapy-related adverse outcomes.
Keywords: Advanced therapy; Biologic agents; Biologics; Crohn's disease; Inflammatory bowel disease; Janus kinase inhibitors; Sequencing; Treatment strategy; Ulcerative colitis.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Imbrizi M has received fees for serving as a speaker and/or an advisory board member for Abbvie, Ferring, Janssen, Nestle, Pfizer and Takeda; Azevedo MFC has received fees for serving as a speaker and/or an advisory board member for Abbvie, Janssen and Takeda; Baima JP has received fees for serving as a speaker and/or an advisory board member for Janssen and AbbVie; Queiroz NSF has served as a speaker and advisory board member of Janssen, Takeda and Abbvie; Parra RS has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, AbbVie, Ferring and Pfizer; Ferreira SDC has received fees for serving as a speaker and/or an advisory board member for Janssen, Takeda, and Pfizer; Sassaki LY has received fees for serving as a speaker and/or an advisory board member for Janssen and AbbVie; Chebli JMF has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, AbbVie, Abbott, and Sandoz.
Figures


References
-
- Zaltman C, Parra RS, Sassaki LY, Santana GO, Ferrari MLA, Miszputen SJ, Amarante HMBS, Kaiser Junior RL, Flores C, Catapani WR, Parente JML, Bafutto M, Ramos O, Gonçalves CD, Guimaraes IM, da Rocha JJR, Feitosa MR, Feres O, Saad-Hossne R, Penna FGC, Cunha PFS, Gomes TN, Nones RB, Faria MAG, Parente MPPD, Scotton AS, Caratin RF, Senra J, Chebli JM. Real-world disease activity and sociodemographic, clinical and treatment characteristics of moderate-to-severe inflammatory bowel disease in Brazil. World J Gastroenterol. 2021;27:208–223. - PMC - PubMed
-
- Noor NM, Bourke A, Subramanian S. Review article: Novel therapies in inflammatory bowel disease - An update for clinicians. Aliment Pharmacol Ther. 2024;60:1244–1260. - PubMed
-
- Dulai PS, Singh S, Jairath V, Wong E, Narula N. Integrating Evidence to Guide Use of Biologics and Small Molecules for Inflammatory Bowel Diseases. Gastroenterology. 2024;166:396–408.e2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

