Gut-liver axis in diabetes: Mechanisms and therapeutic opportunities
- PMID: 40809928
- PMCID: PMC12344363
- DOI: 10.3748/wjg.v31.i29.109090
Gut-liver axis in diabetes: Mechanisms and therapeutic opportunities
Abstract
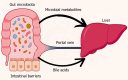
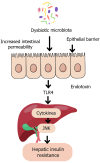
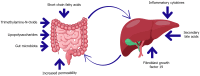
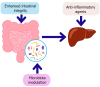
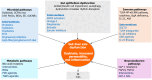
The gut-liver axis represents a complex, bidirectional communication network between the gastrointestinal tract and the liver, playing a central role in maintaining metabolic homeostasis. In diabetes, disruption of this axis, mediated by gut microbiota dysbiosis, impaired intestinal barrier function, and pro-inflammatory signaling, contributes significantly to insulin resistance, hepatic steatosis, and systemic metabolic dysfunction. This review explores the underlying mechanisms by which microbial alterations, increased gut permeability, and inflammatory pathways influence hepatic insulin resistance and glucose metabolism. In addition to established mechanisms, emerging pathways involving neuroendocrine circuits, microbial metabolites, and immune mediators are discussed, offering deeper insight into gut-liver interactions in metabolic disease. The review also outlines therapeutic strategies targeting the gut-liver axis, including microbiota modulation, barrier function enhancement, and anti-inflammatory interventions, emphasizing their potential in advancing diabetes management. A conceptual framework is proposed to integrate these components into a precision medicine approach for metabolic regulation. Key challenges in clinical translation, including patient heterogeneity and the absence of reliable biomarkers to guide treatment decisions are also discussed to inform future research. By linking mechanistic understanding with therapeutic innovation, the review highlights the gut-liver axis as a promising target for personalized diabetes care.
Keywords: Anti-inflammatory; Diabetes; Diabetes management; Gut-liver axis; Intestinal permeability; Metabolic homeostasis; Microbiota.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The author has no conflict of interest to declare.
Figures
Similar articles
-
From Gut to Brain: The roles of intestinal microbiota, immune system, and hormones in intestinal physiology and gut-brain-axis.Mol Cell Endocrinol. 2025 Sep 15;607:112599. doi: 10.1016/j.mce.2025.112599. Epub 2025 Jun 6. Mol Cell Endocrinol. 2025. PMID: 40482955 Review.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Gut Microbiota-Targeted Therapeutics for Metabolic Disorders: Mechanistic Insights into the Synergy of Probiotic-Fermented Herbal Bioactives.Int J Mol Sci. 2025 Jun 7;26(12):5486. doi: 10.3390/ijms26125486. Int J Mol Sci. 2025. PMID: 40564947 Free PMC article. Review.
-
Intestinal inflammation and microbiota modulation impact cochlear function: emerging insights in gut-ear axis.Cell Commun Signal. 2025 Jul 26;23(1):357. doi: 10.1186/s12964-025-02338-1. Cell Commun Signal. 2025. PMID: 40713718 Free PMC article.
-
Understanding crosstalk between the gut and liver microbiome: pathogenesis to therapeutic approaches in liver cancer.Cancer Cell Int. 2025 Jul 29;25(1):291. doi: 10.1186/s12935-025-03840-9. Cancer Cell Int. 2025. PMID: 40730999 Free PMC article. Review.
References
-
- Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. 2021;27:844–855. - PubMed
-
- Zhou M, Lv J, Chen X, Shi Y, Chao G, Zhang S. From gut to liver: Exploring the crosstalk between gut-liver axis and oxidative stress in metabolic dysfunction-associated steatotic liver disease. Ann Hepatol. 2025;30:101777. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

