The Impact of Critical Care Quarantine on the Residual Risk of Unexpected Organ Donor Blood-borne Virus Infection
- PMID: 40810059
- PMCID: PMC12348399
- DOI: 10.1097/TXD.0000000000001843
The Impact of Critical Care Quarantine on the Residual Risk of Unexpected Organ Donor Blood-borne Virus Infection
Abstract
Background: The transmission of undetected infections from organ donors to recipients is a persistent concern in transplantation medicine. Despite nucleic acid testing, some infections, especially those acquired recently, may evade detection. This study aimed to model the effects of critical care interval before screening on the residual risk of undetected infections in organ donors.
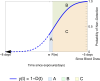
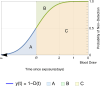
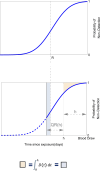
Methods: We modeled the risk of blood-borne virus acquisition in donors using a Poisson process, assuming that the critical care interval carries negligible risk. A continuous probability function was developed using estimates of HIV, hepatitis C virus, and hepatitis B virus incidence, viral doubling rates, and assay performance characteristics from a commonly used triplex nucleic acid screening test.
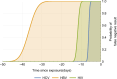
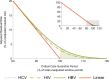
Results: Our quarantine-adjusted window period model showed that longer critical care intervals resulted in a decreased quarantine-adjusted residual risk of undetected infections. This relationship was linear for most assay window periods. For example, a typical critical care interval of 2.7 d reduces the residual risk by 43.5% for hepatitis C virus, 22.9% for HIV, and 7.4% for hepatitis B virus. In some clinical situations, the critical care quarantine effect may outweigh intragroup variations in risk behavior. The model also enabled comparisons of different blood collection times.
Conclusions: The quarantine-adjusted window period model indicates that the critical care interval further reduces the risk of undetected infections in deceased organ donors. This supports and quantifies the impact of screening organ donors as close to retrieval surgery as possible, rather than delaying surgery solely based on the risk of residual infection.
Copyright © 2025 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
References
-
- Kaul DR, Tlusty SM, Michaels MG, et al. Donor-derived hepatitis C in the era of increasing intravenous drug use: a report of the Disease Transmission Advisory Committee. Clin Transplant. 2018;32:e13370. - PubMed
-
- Seed CR, Cheng A, Ismay SL, et al. ; Virology Subcommittee of the National Donor and Product Safety Committee, Australian Red Cross Blood Service. Assessing the accuracy of three viral risk models in predicting the outcome of implementing HIV and HCV NAT donor screening in Australia and the implications for future HBV NAT. Transfusion. 2002;42:1365–1372. - PubMed
-
- Seed CR, Kiely P, Hoad VC, et al. Refining the risk estimate for transfusion-transmission of occult hepatitis B virus. Vox Sang. 2017;112:3–8. - PubMed
-
- Seed CR, Kiely P, Keller AJ. Residual risk of transfusion transmitted human immunodeficiency virus, hepatitis B virus, hepatitis C virus and human T lymphotrophic virus. Intern Med J. 2005;35:592–598. - PubMed
LinkOut - more resources
Full Text Sources

