Diabetes and calcific aortic valve disease: controversy of clinical outcomes in diabetes after aortic valve replacement
- PMID: 40810069
- PMCID: PMC12343241
- DOI: 10.3389/fendo.2025.1577762
Diabetes and calcific aortic valve disease: controversy of clinical outcomes in diabetes after aortic valve replacement
Abstract
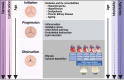
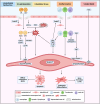
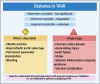
Calcific aortic valve disease (CAVD) is a progressive disease, of which the 2-year mortality is >50% for symptomatic aortic valve stenosis unless transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR) is performed promptly. The prevalence of diabetes among CAVD has increased rapidly in recent years. The combination of diabetes with its cardio-renal and metabolic comorbidities, such as hypertension, hyperlipidemia, chronic kidney disease, and ageing, accelerated the progression of CAVD and increased the subsequent needs for aortic valve replacement. Clinical data regarding the impact of diabetes on outcomes of patients undergoing TAVR or SAVR have exhibited inconsistent results. Compared with non-diabetes, the short-term mortality after TAVR was not significant in diabetes, while the mid-term mortality differed from different cohorts. Although there were worse mid-term and long-term mortalities after SAVR in diabetes, the short-term mortality in diabetes was disputable. As for complications, there were common worse manifestations with coronary heart disease, acute kidney injury, heart failure, and systemic inflammatory response syndrome in diabetes undergoing TAVR or SAVR. Moreover, diabetes was one of the risk factors for deterioration of bioprosthetic aortic valves, leading to increased long-term mortality. Based on the efficacy for CAVD and atherosclerotic cardiovascular disease, glucose-lowering medications might have potential to inhibit deterioration of bioprosthetic aortic valves independent of glucose control.
Keywords: calcific aortic valve disease; deterioration of bioprosthetic aortic valve; diabetes; surgical aortic valve replacement; transcatheter aortic valve replacement.
Copyright © 2025 Liu and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Clinical Characteristics and Outcomes of Patients Undergoing 3 Aortic Valve Interventions: The THIRD Multicenter Registry.JACC Cardiovasc Interv. 2025 Jan 13;18(1):103-115. doi: 10.1016/j.jcin.2024.10.037. JACC Cardiovasc Interv. 2025. PMID: 39814484
-
Diabetes is associated with a higher incidence of short-term mortality risk and readmission in patients who undergo surgical but not transcatheter aortic valve replacement.Open Heart. 2025 Jan 11;12(1):e003019. doi: 10.1136/openhrt-2024-003019. Open Heart. 2025. PMID: 39800433 Free PMC article.
-
Meta-analysis of longitudinal comparison of transcatheter versus surgical aortic valve replacement in patients at low to intermediate surgical risk.Int J Surg. 2024 Dec 1;110(12):8097-8106. doi: 10.1097/JS9.0000000000002158. Int J Surg. 2024. PMID: 39806748 Free PMC article.
-
Transcatheter Aortic Valve Replacement is Associated with Comparable Clinical Outcomes to Open Aortic Valve Surgery but with a Reduced Length of In-Patient Hospital Stay: A Systematic Review and Meta-Analysis of Randomised Trials.Heart Lung Circ. 2017 Mar;26(3):285-295. doi: 10.1016/j.hlc.2016.07.011. Epub 2016 Aug 29. Heart Lung Circ. 2017. PMID: 27646577
-
Valve-in-valve transcatheter aortic valve replacement versus redo surgical aortic valve replacement: A systematic review and meta-analysis.J Card Surg. 2021 Jul;36(7):2486-2495. doi: 10.1111/jocs.15546. Epub 2021 Apr 2. J Card Surg. 2021. PMID: 33797799
References
-
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. (2011) 124:1783–91. doi: 10.1161/circulationaha.110.006767, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

