Viral and nonviral nanocarriers for in vivo CRISPR-based gene editing
- PMID: 40810089
- PMCID: PMC12341846
- DOI: 10.1007/s12274-024-6748-5
Viral and nonviral nanocarriers for in vivo CRISPR-based gene editing
Abstract
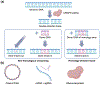
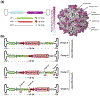
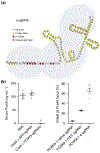
The continued development of clustered regularly interspaced short palindromic repeats (CRISPR) technology has the potential to greatly impact clinical medicine, particularly for disease diagnosis and treatment. Despite high demand for the in vivo delivery of CRISPR-based therapies, significant challenges persist. These include rapid degradation by enzymes, inefficient disease site targeting, and the risk of undesired off-target outcomes. Nanoparticulate platforms, with their tailorable properties, have been engineered to efficiently package CRISPR payloads in various formats, including as plasmid DNA, mRNA, and ribonucleoprotein complexes, for in vivo delivery. Among them, recombinant adeno-associated viruses, virus-like particles, and lipid nanoparticles have displayed exceptional promise. This review will discuss the development of these and other nanocarriers for in vivo CRISPR-based genome editing.
Keywords: clustered regularly interspaced short palindromic repeats (CRISPR); in vivo gene editing; lipid nanoparticle (LNP); nanocarriers; recombinant adeno-associated viruses (rAAVs); virus-like particle (VLP).
Figures

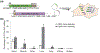




Similar articles
-
Trojan Horse-Like Vehicles for CRISPR-Cas Delivery: Engineering Extracellular Vesicles and Virus-Like Particles for Precision Gene Editing in Cystic Fibrosis.Hum Gene Ther. 2025 Aug;36(15-16):1021-1052. doi: 10.1089/hum.2024.258. Epub 2025 Apr 28. Hum Gene Ther. 2025. PMID: 40295092 Review.
-
Improving the use of CRISPR/Cas9 gene editing machinery as a cancer therapeutic tool with the help of nanomedicine.3 Biotech. 2025 Jan;15(1):17. doi: 10.1007/s13205-024-04186-1. Epub 2024 Dec 19. 3 Biotech. 2025. PMID: 39711922 Review.
-
Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review.Cells. 2025 Jul 23;14(15):1136. doi: 10.3390/cells14151136. Cells. 2025. PMID: 40801569 Free PMC article. Review.
-
Applying CRISPR Technologies for the Treatment of Human Herpesvirus Infections: A Scoping Review.Pathogens. 2025 Jul 1;14(7):654. doi: 10.3390/pathogens14070654. Pathogens. 2025. PMID: 40732701 Free PMC article. Review.
-
Cell-Penetrating Peptides and CRISPR-Cas9: A Combined Strategy for Human Genetic Disease Therapy.Hum Gene Ther. 2024 Oct;35(19-20):781-797. doi: 10.1089/hum.2024.020. Hum Gene Ther. 2024. PMID: 39276086 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
