Endothelial c-Maf prevents MASLD-like liver fibrosis by regulating chromatin accessibility to suppress pathogenic microvascular cell subsets
- PMID: 40810103
- PMCID: PMC12341620
- DOI: 10.1016/j.jhepr.2025.101475
Endothelial c-Maf prevents MASLD-like liver fibrosis by regulating chromatin accessibility to suppress pathogenic microvascular cell subsets
Abstract
Background & aims: Liver sinusoidal endothelial cells (LSECs) are highly specialized components of the hepatic vascular niche, regulating liver function and disease pathogenesis through angiocrine signaling. Recently, we identified GATA4 as a key transcription factor controlling LSEC development and protecting against liver fibrosis. As the transcription factor c-Maf was strongly downregulated in Gata4-deficient LSECs, we hypothesized that c-Maf might be an important downstream effector of GATA4 in LSEC differentiation and liver fibrogenesis.
Methods: Clec4g-iCre/Maf fl/fl (Maf LSEC-KO ) mice with LSEC-specific Maf deficiency were generated and liver tissue was analyzed histologically. LSECs were isolated for bulk RNA-seq, ATAC-seq, and single-cell (sc) RNA-seq analysis. Maf LSEC-KO livers were analyzed after MASH diet feeding. The expression of MAF and its targets was analyzed in published human scRNA-seq data.
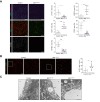
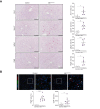
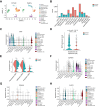
Results: Endothelial Maf deficiency resulted in perisinusoidal liver fibrosis (Sirius red 0.46% vs. 2.92%; p <0.05) without affecting metabolic liver zonation, accompanied by a switch from sinusoidal to continuous endothelial cell identity, which was aggravated upon MASH diet feeding (p <0.01). Furthermore, endothelial Maf deficiency caused LSEC proliferation (p <0.05) and expression of profibrotic angiocrine factors including Pdgfb, Igfbp5, Flrt2, and Cxcl12, among which FLRT2 (p <0.01) and CXCL12 (p <0.001) activated hepatic stellate cells in vitro. scRNA-seq revealed replacement of zonated LSEC subpopulations with capillarized, proliferative, sprouting and secretory endothelial cell subsets that promote liver fibrogenesis and angiogenesis. This fundamental dysregulation of LSEC gene expression and differentiation was caused by changes in chromatin accessibility and transcription factor activity following loss of Maf. Notably, endothelial MAF expression was also significantly reduced in human cirrhotic livers (p <0.0001).
Conclusions: Hepatic endothelial c-Maf protects against metabolic dysfunction-associated steatohepatitis-like liver fibrosis and regulates endothelial differentiation and zonation by controlling chromatin opening.
Impact and implications: This work builds on the known importance of liver sinusoidal endothelial cells in liver function and disease. Here, transcription factor c-Maf is identified as a master regulator in maintaining normal differentiation and zonation of liver sinusoidal endothelial cells, thereby protecting against the development of liver fibrosis/cirrhosis. The findings are significant for researchers and clinicians focusing on liver disease, as they suggest potential new targets for therapeutic intervention. These findings could instruct the development of novel preventive treatment options and antifibrotic therapy regimens as well as liver repair strategies, benefiting patients, clinicians and policy makers in the management of liver disease.
Keywords: ATAC-Seq analysis; Capillarization; Cirrhosis; Liver sinusoidal endothelial cells (LSEC); Single-cell RNA-Seq analysis.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

