Machine learning-driven optimization of culture conditions and media components to mitigate charge heterogeneity in monoclonal antibody production: current advances and future perspectives
- PMID: 40810344
- PMCID: PMC12355708
- DOI: 10.1080/19420862.2025.2547084
Machine learning-driven optimization of culture conditions and media components to mitigate charge heterogeneity in monoclonal antibody production: current advances and future perspectives
Abstract
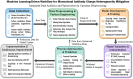
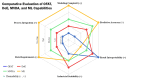
Charge heterogeneity in monoclonal antibodies (mAbs), caused by post-translational modifications, remains a substantial obstacle to ensuring consistent, stable, and effective therapeutics. Conventional optimization techniques, such as one-factor-at-a-time and design of experiments, often fail to capture the complex, nonlinear interactions between culture parameters (e.g. pH, temperature, duration) and medium components (e.g. glucose, metal ions, amino acids). This review highlights machine learning (ML) as a powerful approach for modeling these relationships and forecasting charge variant profiles in CHO cell-based mAb process development. We summarize supervised learning and regression methods used to link process conditions with charge heterogeneity and present case studies showing ML's role in reducing acidic and basic variants. We also discuss challenges related to data quality, model interpretability, scalability, and regulatory compliance. Finally, we propose a roadmap for adaptive, ML-driven optimization strategies for bioprocess development, aligned with Quality-by-Design principles.
Keywords: Monoclonal antibody; bioprocessing; charge heterogeneity; machine learning; medium optimization; quality by design.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Recent advances in culture medium design for enhanced production of monoclonal antibodies in CHO cells: A comparative study of machine learning and systems biology approaches.Biotechnol Adv. 2025 Jan-Feb;78:108480. doi: 10.1016/j.biotechadv.2024.108480. Epub 2024 Nov 19. Biotechnol Adv. 2025. PMID: 39571767 Review.
-
Electrophoresis.2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36251838 Free Books & Documents.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Machine learning-based identification of key biotic and abiotic drivers of mineral weathering rate in a complex enhanced weathering experiment.Open Res Eur. 2025 Jul 3;5:71. doi: 10.12688/openreseurope.19252.2. eCollection 2025. Open Res Eur. 2025. PMID: 40792254 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Kavoni H, Savizi ISP, Lewis NE, Shojaosadati SA.. Recent advances in culture medium design for enhanced production of monoclonal antibodies in CHO cells: a comparative study of machine learning and systems biology approaches. Biotechnol Adv. 2025;78:108480. doi: 10.1016/j.biotechadv.2024.108480. - DOI - PMC - PubMed
-
- Malhotra N, Chahal A, Jain A, Sharma P, Saini P, Hasan MR, Narang J. Monoclonal antibodies: purification, application in conventional methods and cutting edge technology. Biomed Mater Devices. 2024;3(1):367–20. doi: 10.1007/s44174-024-00203-2. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
