Connectivity as a universal predictor of tau progression in atypical Alzheimer's disease
- PMID: 40810361
- PMCID: PMC12588720
- DOI: 10.1093/brain/awaf279
Connectivity as a universal predictor of tau progression in atypical Alzheimer's disease
Abstract
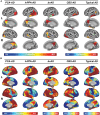
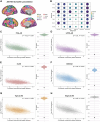
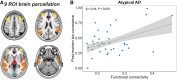
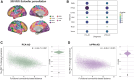
The link between regional tau load and clinical manifestation of Alzheimer's disease (AD) highlights the importance of characterizing spatial tau distribution across disease variants. In typical (memory-predominant) AD, the spatial progression of tau pathology mirrors the functional connections from temporal lobe epicentres. However, given the limited spatial heterogeneity of tau in typical AD, atypical (non-amnestic-predominant) AD variants with distinct tau patterns provide a key opportunity to investigate the universality of connectivity as a scaffold for tau progression. In this large-scale, multicentre study across 14 international sites, we included cross-sectional tau-PET data from 320 individuals with atypical AD (n = 139 posterior cortical atrophy/PCA-AD; n = 103 logopenic variant primary progressive aphasia/lvPPA-AD; n = 35 behavioural variant AD/bvAD; n = 43 corticobasal syndrome/CBS-AD), with a subset of individuals (n = 78) having longitudinal tau-PET data. Additionally, as an independent sample, we included regional post-mortem tau stainings from 93 atypical AD patients from two sites (n = 19 PCA-AD, n = 32 lvPPA-AD, n = 23 bvAD, n = 19 CBS-AD). Gaussian mixture modelling was used to harmonize different tau-PET tracers by transforming tau-PET standardized uptake value ratios to tau positivity probabilities (a uniform scale ranging from 0% to 100%). Using linear regression, we assessed whether brain regions with stronger resting-state functional MRI-based functional connectivity, derived from healthy elderly controls in the Alzheimer's Disease Neuroimaging Initiative (ADNI), showed greater covariance in cross-sectional and longitudinal tau-PET and post-mortem tau pathology. Furthermore, we examined whether functional connectivity of tau-PET epicentres (i.e. the top 5% of regions with the highest baseline tau load) and tau-PET accumulation epicentres (i.e. the top 5% of regions with the highest tau accumulation rates) was associated with cross-sectional and longitudinal tau patterns. Our findings show that tau-PET epicentres aligned with clinical variants, e.g. a visual network predominant pattern in PCA-AD ('visual AD') and left-hemispheric temporal predominance, particularly within the language network, in lvPPA-AD ('language AD'). Moreover, more strongly functionally connected regions showed correlated concurrent tau-PET levels (confirmed with post-mortem data) and tau-PET accumulation rates. The functional connectivity profile of tau-PET epicentres and accumulation epicentres corresponded to tau-PET progression patterns, with higher tau-PET levels and accumulation rates in functionally close regions, and lower tau-PET levels and accumulation rates in functionally distant regions. Our data are consistent with the hypothesis that tau propagation occurs along functional connections originating from local epicentres, across all AD clinical variants. Since tau proteinopathy is a major driver of neurodegeneration and cognitive decline, this finding may advance personalized medicine and participant-specific end points in clinical trials.
Keywords: PET; atypical Alzheimer's disease; connectivity; fMRI; heterogeneity; tau.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
C.G. (Amsterdam) is supported by a Dementia Fellowship grant from ZonMW (10510022110010). Y.A.L.P. (Amsterdam) has received funding from the Dutch Brain Foundation, ZonMW, NWO and the Mooiste Contact Fonds (both paid to her institution). N.F. (Munich) has received funding from the Alzheimer's Association, Bright Focus Foundation, Alzheimer Forschung Initiative, Schick Foundation, Avid Radiopharmaceuticals, Legerlotz Foundation and has received speaker honoraria from Eisai, Life Molecular Imaging, GE Healthcare and consulting honoraria from MSD. Projects of R.O. (Amsterdam) received support of the European Research Council, ZonMw, NWO, National Institute of Health, Alzheimer Association, Alzheimer Nederland, Stichting Dioraphte, Cure Alzheimer's fund, Health Holland, ERA PerMed, Alzheimerfonden, Hjarnfonden, Avid Radiopharmaceuticals, Janssen Research & Development, Roche, Quanterix and Optina Diagnostics. R.O. was a speaker at symposia organized by GE healthcare. R.O. is an advisory board member for Asceneuron, Bristol Myers Squibb and Biogen. All the aforementioned has been paid to the institutions. R.O. is part of the editorial board of Alzheimer's Research & Therapy and the European Journal of Nuclear Medicine and Molecular Imaging. M.M. (Cambridge) provides consultancy for Astex Pharmaceuticals (unrelated to this work). J.B.R. (Cambridge) reports: consultancy for Asceneuron, Alector, Astronautx, Astex, CumulusNeuro, ClinicalInk, Curasen, Eisai, Ferrer, Prevail and SVHealth, unrelated to the current work. A.D. (Cologne) reports: research support by Siemens Healthineers, Life Molecular Imaging, GE Healthcare, AVID Radiopharmaceuticals, Sofie, Eisai, Novartis/AAA, Ariceum Therapeutics; Speaker Honorary/Advisory Boards: Siemens Healthineers, Sanofi, GE Healthcare, Biogen, Novo Nordisk, Invicro, Novartis/AAA, Bayer Vital, Lilly; Stock: Siemens Healthineers, Lantheus Holding, Structured therapeutics, Lilly: patents: patent for 18F-JK-PSMA- 7 (Patent No.: EP3765097A1; Date of patent: 20 January, 2021). T.vE. (Cologne) reports: advisory boards (ICON, Bial, Lundbeck Foundation), honoraria (Eisai, International Society for Parkinson and Movement Disorders, Korean Movement Disorders Society), consultancies (GT Gain Therapeutics SA, INSERM), grants (German Research Foundation, Humboldt Foundation, Brandau-Laibach Foundation). H.B. (Leipzig) received reader honoraria from Life Molecular Imaging, speaker honoraria from Novartis/AAA and IBA, dosing committee honoraria from Pharmtrace, and consulting honoraria from Lilly. O.H. (Lund) is an employee of Lund University and Eli Lilly. R.S. (Lund) has received speaker honoraria from Roche and Triolab. B.D.C.B. (Mayo) has received funding from Alzheimer Nederland (#WE.15-2019-13, #WE.03-2021-15, #WE.06-2023-01). P.R.N. (McGill) participated in Advisory Board for Roche, Novo Nordics and Cerveau (outside submitted work). J.T. (McGill) has served as a paid consultant for Neurotorium and for Alzheon Inc. M.B. (Munich) received speaker honoraria from Roche, Iba, GE Healthcare and Life Molecular Imaging, is an active advisor of MIAC, and advised GE Healthcare Life Molecular Imaging. J.L. (Munich) reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA, Zambon, Esteve, Merck and Roche, consulting fees from Axon Neuroscience, EISAI and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers and is inventor in a patent ‘Oral Phenylbutyrate for Treatment of Human 4-Repeat Tauopathies’ (PCT/EP2024/053388) filed by LMU Munich. In addition, he reports compensation for serving as chief medical officer for MODAG GmbH, is beneficiary of the phantom share program of MODAG GmbH and is inventor of a patent ‘Pharmaceutical Composition and Methods of Use’ (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work. D.M.C. (UCL) reports a paid consultancy with Perceptive Imaging. J.M.S. (UCL) has received research funding and PET tracer from AVID Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) and Alliance Medical; has consulted for Roche, Eli Lilly, Biogen, AVID, Merck and GE; and received royalties from Oxford University Press and Henry Stewart Talks. He is Chief Medical Officer for Alzheimer’s Research UK. R.L.J. (UCSF) consulted for GE Healthcare. G.D.R. (UCSF) receives research support from Avid Radiopharmaceuticals, GE Healthcare, Life Molecular Imaging, Genentech. He has served as a paid consultant for Alector, Eli Lilly, Johnson & Johnson, Merck. He is a member of the AD Therapeutics Workgroup and an Associate Editor for JAMA Neurology. E.B.L. (UPENN) has received consulting fees from Lilly and Wavebreak Therapeutics. R.S.O. (Yale) reports grants for clinical trials from Cognition Therapeutics and Bristol-Myers Squibb outside of the submitted work. The other authors report no competing interests.
Figures


Comment in
-
Wherever tau pathology starts, propagation will follow brain connections.Brain. 2025 Nov 4;148(11):3787-3788. doi: 10.1093/brain/awaf350. Brain. 2025. PMID: 40991746 No abstract available.
References
-
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Deutsche Forschungsgemeinschaft
- AF-994229/Swedish Alzheimer Foundation
- AF-980907/Swedish Alzheimer Foundation
- Alzheimer's Society and Alzheimer's Research UK
- MR/T033371/1/MRC_/Medical Research Council/United Kingdom
- P30 AG066444/AG/NIA NIH HHS/United States
- 2022-0231)/Knut and Alice Wallenberg Foundation
- R21 AG080588/NH/NIH HHS/United States
- R01 AG043434/AG/NIA NIH HHS/United States
- National Institute for Health
- AVID Radiopharmaceuticals
- 2019-02397/Swedish Research Council
- 2020-O000028/Skåne University Hospital Foundation
- R01 DC014296/DC/NIDCD NIH HHS/United States
- 2022-Projekt0080/Swedish federal government under the ALF agreement
- ID 403161218/German Research Foundation
- 2022-1259/Regionalt Forskningsstöd
- R21 AG080588/AG/NIA NIH HHS/United States
- P30AG021342/Adult Children Study
- CRC1451-C04/German Research Foundation
- eHealthSax Initiative of the Sächsische Aufbaubank
- ALFGBG-71320/Swedish federal government under the ALF agreement
- K23 AG065450/NH/NIH HHS/United States
- P01 AG084497/AG/NIA NIH HHS/United States
- Saxon state parliament
- 2019-AACSF-644153/ALZ/Alzheimer's Association/United States
- ZEN24-1069572/ALZ/Alzheimer's Association/United States
- 390857198/Munich Cluster for Systems Neurology
- AARG-22-926144/ALZ/Alzheimer's Association/United States
- P50 AG005134/NH/NIH HHS/United States
- RFN 152985/CAPMC/ CIHR/Canada
- Strategic Research Area MultiPark
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG085377/NH/NIH HHS/United States
- NIRP-12-259245/ALZ/Alzheimer's Association/United States
- K01 AG084820/NH/NIH HHS/United States
- UK Dementia Research Institute
- 2022-00775/Swedish Research Council
- FO2021-0293/Swedish Brain Foundation
- R01AG052560/Adult Children Study
- UK Medical Research Council
- P30 AG066508/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- R01 AG062276/AG/NIA NIH HHS/United States
- #329109473/German Research Foundation
- Lund University
- 220258/WT_/Wellcome Trust/United Kingdom
- P01 AG026276/AG/NIA NIH HHS/United States
- National Institute for Health Research University College London Hospitals Biomedical Research Centre
- P50 AG047270/AG/NIA NIH HHS/United States
- MC_UU_00030/14/MRC_/Medical Research Council/United Kingdom
- R01 DC014296/NH/NIH HHS/United States
- K23 AG059919/AG/NIA NIH HHS/United States
- 21010CB/Alzheimer Forschung Initiative
- 2023-00356/Swedish Research Council
- P30AG066508/Adult Children Study
- Life Molecular Imaging CMC
- K23AG059919/Adult Children Study
- GHR Foundation
- P30 AG062422/AG/NIA NIH HHS/United States
- GE Healthcare
- ADG-101096455/ERC_/European Research Council/International
- FO2023-0163/Swedish Brain Foundation
- Massachusetts General Hospital
- Brain Canada Foundation
- RF1 NS131395/NS/NINDS NIH HHS/United States
- P30 AG021342/AG/NIA NIH HHS/United States
- ADG-101053962/ERC_/European Research Council/International
- R01 EB009352/EB/NIBIB NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- R01 AG081249/NH/NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- SG-666374-UK/ALZ/Alzheimer's Association/United States
- P30 NS04805/Adult Children Study
- NIRG-12-92090/ALZ/Alzheimer's Association/United States
- R01 AG085377/AG/NIA NIH HHS/United States
- MOP-11-51-31-team 1/Canadian Consortium of Neurodegeneration and Aging
- MOP-11-51-31/CAPMC/ CIHR/Canada
- Weston Brain Institute
- P01 AG066597/AG/NIA NIH HHS/United States
- P30 AG062421/NH/NIH HHS/United States
- P30AG047270/Adult Children Study
- SG-23-1061717/ALZ/Alzheimer's Association/United States
- Rönström Family Foundation
- R35 AG072362/AG/NIA NIH HHS/United States
- 2022-Projekt0107/Swedish federal government under the ALF agreement
- Colin J. Adair Charitable Foundation
- R35-AG072362/NIH/NIA
- Cure Alzheimer's fund
- P01AG084497/Penn Alzheimer's Disease Research Center
- 1412/22/Parkinson Foundation of Sweden
- WASP/DDLS22-066/Swedish Brain Foundation
- UL1 TR000448/TR/NCATS NIH HHS/United States
- K23 DC016912/NH/NIH HHS/United States
- 2022-01018/Swedish Research Council
- K23 AG065450/AG/NIA NIH HHS/United States
- NIHR203312/NIHR Cambridge Biomedical Research Centre
- R01 AG081249/AG/NIA NIH HHS/United States
- WE.03-2021-12cb/Alzheimer Nederland
- P01 AG03991/Healthy Aging and Senile Dementia
- K23 DC016912/DC/NIDCD NIH HHS/United States
- R01-AG50603/NH/NIH HHS/United States
- R01AG062276/Adult Children Study
- WT_/Wellcome Trust/United Kingdom
- RF1 NS131395/NH/NIH HHS/United States
- P01AG066597/Penn Alzheimer's Disease Research Center
- Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse
- P30-AG062422/NIH/NIA
- CurePSP
- R01 AG045611/AG/NIA NIH HHS/United States
- ERAPERMED2021-184/ERA PerMed
- SCHR 774/5-1/German Research Foundation
- R01 AG052560/AG/NIA NIH HHS/United States
- ARUK-RADF2021A-010/Race Against Dementia Alzheimer's Research UK
- 159815/CAPMC/ CIHR/Canada
- Care Research University College London Hospitals Biomedical Research Centre
- R01-AG045611/NIH/NIA
- 162303/CAPMC/ CIHR/Canada
- Dementias Platform UK
- DR 445/9-1/German Research Foundation
- P30 AG072979/AG/NIA NIH HHS/United States
- 2020-VICO-279314/Fonds de Recherche du Québec-Santé
- K01 AG084820/AG/NIA NIH HHS/United States
- R01 AG054519/AG/NIA NIH HHS/United States
- 2021-02219/Swedish Research Council
- R01 AG050603/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

