Human Pluripotent Stem Cell-Derived Skeletal Muscle Organoid Model of Aging-Induced Sarcopenia
- PMID: 40810394
- PMCID: PMC12351639
- DOI: 10.1002/jcsm.70045
Human Pluripotent Stem Cell-Derived Skeletal Muscle Organoid Model of Aging-Induced Sarcopenia
Abstract
Background: Sarcopenia is defined by the age-related loss of muscle mass and function, with an impaired regenerative capacity of satellite cells (SCs). Despite their recognized importance in muscle regeneration, human model-based studies on SCs in sarcopenia are still lacking, limiting our understanding of their role in age-related muscle loss. Here, we aimed to develop a sarcopenia model using human pluripotent stem cells (hPSCs)-derived skeletal muscle organoids (hSkMOs) and prevent the sarcopenia progression by testosterone treatment.
Methods: The 3D hSkMOs were generated from hPSC and exhibited structurally and functionally mature muscle fibres and spinal-derived neurons including motor neurons and interneurons. The proportion of muscle and the diameter of muscle fibres were assessed. To investigate the acute pro-inflammatory response and intrinsic regenerative capacity of hSkMOs, we induced sarcopenia-like conditions by TNF-α treatment for 2 days and analysed. To model aging-induced sarcopenia and investigate the preventive effect of testosterone, chronic TNF-α treatment was applied, followed by testosterone administration. Histological, biochemical, molecular and electrophysiological analyses were conducted in various experiments.
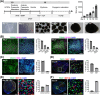
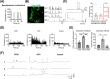
Result: We employed a stepwise differentiation protocol from 2D paraxial mesodermal induction to 3D myogenic specification, concluding with a maturation culture system. We observed that the majority of cells were T/BRA- and TBX6-positive (+) paraxial mesodermal progenitors (T/BRA+, 82.04%; TBX6+, 78.18%), whereas the neuromesodermal progenitors demonstrated a relatively low proportion (T/BRA+/SOX2+, 15.91%; TBX6+/SOX2+, 11.45%). Single-nucleus RNA-sequencing and extensive immunohistochemistry confirmed the presence of the myogenic lineage cell types (myogenic progenitors/SCs, myocytes, muscle fibres) and the neural lineage cell types (spinal-derived interneurons, motor neurons, glial cells, Schwann cells). Additionally, the growth of MyHC+ muscle fibres reached twice the thickness on Day 100 compared to that on Day 50 (p < 0.0001). We subjected them to TNF-α treatment and analysed. Western blot analysis confirmed that TNF-α/NF-κB pathway associated factors such as NF-κB p65, IκB-α and AKT were highly phosphorylated (p < 0.05, p < 0.001). The administration of testosterone increased the proportion of activated SCs (PAX7+/MYOD+, 7.97%; PAX7+/Ki67+, 7.03%) compared to the TNF-α group (PAX7+/MYOD+, 2.29%; PAX7+/Ki67+, 2.07%, p < 0.001). The administration of testosterone increased the Cross-Sectional-Area (987.1 μm2) compared to the TNF-α group (644.7 μm2, p < 0.01).
Conclusions: We successfully developed a hSkMOs to demonstrate the structural maturity of the skeletal muscle and its functional interaction with spinal-derived interneurons and motor neurons. Furthermore, we demonstrated that our hSkMOs are useful for modelling aging-induced sarcopenia and providing a valuable platform for testing therapeutic interventions.
Keywords: aging; human pluripotent stem cell; human skeletal muscle organoid; sarcopenia; satellite cell; testosterone.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Ajou University School of Medicine has filed a provisional patent application that covers the generation of hSkMOs.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

