Acute effects of butyrate on intestinal permeability in patients with irritable bowel syndrome assessed by a novel colonoscopy research model
- PMID: 40810534
- PMCID: PMC12355679
- DOI: 10.1080/19490976.2025.2545414
Acute effects of butyrate on intestinal permeability in patients with irritable bowel syndrome assessed by a novel colonoscopy research model
Abstract
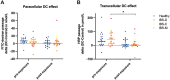
Irritable bowel syndrome (IBS) is a prevalent gastrointestinal disorder for which effective treatment strategies are insufficient. Butyrate, a microbiota-derived short-chain fatty acid believed to strengthen the intestinal barrier function, might be a potential new treatment option. This study aimed to investigate potential protective effects of acute in vivo butyrate exposure on intestinal barrier function in healthy subjects and patients with IBS. For this, we used an experimental colonoscopy-perfusion model for colon-specific butyrate delivery and adequate tissue sampling. Seventeen IBS and 17 healthy subjects underwent a colonoscopy procedure exposing a predefined colonic area to 100 mmol/L butyrate for 90 min in vivo. Mucosal biopsies collected pre- and post-butyrate exposure were stimulated in Ussing chambers with/without sodium deoxycholate (DC) to induce intestinal hyperpermeability. Intestinal permeability was measured by fluorescein isothiocyanate-dextran and horseradish peroxidase passage. DC-stimulation significantly increased para- and transcellular permeability in biopsies collected pre-butyrate exposure. DC-induced transcellular hyperpermeability was significantly alleviated in biopsies collected post-butyrate exposure compared to pre-exposure in patients with IBS (p = 0.034). In conclusion, we established a colonoscopy research model for colon-specific delivery and sampling and demonstrated acute protective effects of butyrate on transcellular intestinal permeability in patients with IBS. The results support butyrate's potential role in novel treatment strategies in IBS. Clinicaltrials.gov number: NCT05249023.
Keywords: IBS; Ussing chamber; in vivo; intestinal barrier function.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology. 2021;160(1):99–114.e3. doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
-
- Bednarska O, Walter SA, Casado-Bedmar M, Ström M, Salvo-Romero E, Vicario M, Mayer EA, Keita ÅV. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology. 2017;153(4):948–960.e3. doi: 10.1053/j.gastro.2017.06.051. - DOI - PMC - PubMed
-
- Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201. doi: 10.1136/gut.2007.140806. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
