Virological and Immunological Outcomes of Combined Therapeutic Interventions and Dendritic Cell Therapy in People With HIV
- PMID: 40810569
- PMCID: PMC12614972
- DOI: 10.1093/infdis/jiaf430
Virological and Immunological Outcomes of Combined Therapeutic Interventions and Dendritic Cell Therapy in People With HIV
Abstract
Background: Except for hematopoietic cell transplantation, therapeutic-driven HIV-1 curative approaches have been unsuccessful. Here, we describe a 2-step randomized clinical trial designed to evaluate the safety and impact of individual and combinatorial therapeutic strategies on changes in peripheral and gut mucosal HIV reservoirs, immune activation and function in people with HIV with chronic disease and high CD4 T-cell nadirs.
Methods: Thirty participants were enrolled and randomized equally into 6 study arms based on treatments with either standard antiretroviral therapy (ART) alone or a combination of candidate anti-HIV reservoir strategies that included ART intensification, auranofin (an apoptotic-inducer antirheumatic drug), nicotinamide (vitamin B3), and a personalized dendritic cell therapy.
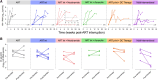
Results: After an analytical treatment interruption post intervention, all eligible participants rebounded within 14 weeks, except for 1 participant with rebound detected at 84 weeks and 2 participants receiving all combined therapies maintained viral loads below 1000 copies/mL through the study period. These posttreatment controllers all received nicotinamide containing regimens and exhibited immune cellular epigenetic age reversal.
Conclusions: This proof-of-concept clinical trial demonstrates the safety of multiple interventions with distinct antireservoir activities in people with HIV and argues for continued investigation of both single and combinatorial interventions towards posttherapy HIV control.
Keywords: CCR5; antiretroviral therapy (ART); cure; immunotherapy; nicotinamide.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . R. S. D. serves on advisory boards for GSK, ViiV Healthcare, Jansen & Jansen, Gilead, and AbbVie. L. C. N. serves as a scientific advisor to AbbVie and ViiV Healthcare, serves on the board of Cytodyn, and has financial interests and serves as a scientific advisor in Ledidi AS, all for work unrelated to this study. All other remaining authors have declared that no competing interests exist. I. L. S., A. S., and R. S. D. have a patent application for personalized dendritic cell therapy. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol 2016; 14:55–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 441817/2018-1/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- UM1 AI164559/AI/NIAID NIH HHS/United States
- 20/10396-2/Fundação de Apoio à Pesquisa do Estado de São Paulo
- CNPq-454700-2014-8/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 19/17461-7/Fundação de Apoio à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources
Medical
Research Materials

