Mitochondrial Quality Control: Insights into Intracerebral Hemorrhage
- PMID: 40810912
- PMCID: PMC12354944
- DOI: 10.1007/s10571-025-01599-1
Mitochondrial Quality Control: Insights into Intracerebral Hemorrhage
Abstract
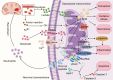
Mitochondrial dysfunction has been identified as a key factor in the pathophysiological changes associated with intracerebral hemorrhage (ICH). As the core of intracellular energy metabolism, mitochondrial homeostasis is highly dependent on the precise regulation of its mitochondrial quality control (MtQC) system. After ICH, dysfunctional mitochondria lead to impaired oxidative phosphorylation and cellular bioenergetic stress, inducing oxidative stress, inflammatory responses, and programmed cell death, further exacerbating cellular damage. To counteract this injury, cells activate a series of MtQC mechanisms for compensatory repair, including mitochondrial dynamics, mitochondrial biogenesis, mitophagy, and intercellular mitochondrial transfer. These stringent mechanisms help maintain the mitochondrial network, restore the integrity of mitochondrial structural and functional integrity, improve neural function, and mitigate brain injury. In this review, we discuss key evidence regarding the role of mitochondrial dysfunction in ICH, focusing on the MtQC mechanisms involved in ICH. We also summarize potential therapeutic strategies targeting MtQC to intervene in ICH, providing valuable insights for clinical applications.
Keywords: Intercellular mitochondrial transfer; Intracerebral hemorrhage; Mitochondrial dynamics; Mitochondrial dysfunction; Mitochondrial quality control; Mitophagy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests. Ethical Approval: Not applicable. Consent to Participate: Not applicable.
Figures

Similar articles
-
Mitochondrial dynamics dysfunction and neurodevelopmental disorders: From pathological mechanisms to clinical translation.Neural Regen Res. 2025 Jun 19. doi: 10.4103/NRR.NRR-D-24-01422. Online ahead of print. Neural Regen Res. 2025. PMID: 40537021
-
Impaired microglial glycolysis promotes inflammatory responses after intracerebral haemorrhage via HK2-dependent mitochondrial dysfunction.J Adv Res. 2025 Jul;73:575-591. doi: 10.1016/j.jare.2024.08.016. Epub 2024 Aug 13. J Adv Res. 2025. PMID: 39142439 Free PMC article.
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
Excitotoxicity, Oxytosis/Ferroptosis, and Neurodegeneration: Emerging Insights into Mitochondrial Mechanisms.Aging Dis. 2024 Aug 1;16(5):2504-2543. doi: 10.14336/AD.2024.0125-1. Aging Dis. 2024. PMID: 39122453 Free PMC article. Review.
-
Mitochondrial quality control in hematopoietic stem cells: mechanisms, implications, and therapeutic opportunities.Stem Cell Res Ther. 2025 Apr 15;16(1):180. doi: 10.1186/s13287-025-04304-7. Stem Cell Res Ther. 2025. PMID: 40234908 Free PMC article. Review.
References
-
- Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia M, Misso G (2020) Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol 98:139–153. 10.1016/j.semcdb.2019.05.022 - PubMed
-
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 33(9):994–1010. 10.1002/embj.201386030 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

