Immunoglobulin G Receptors (FcγR), in Addition to Target-Antigen and Neonatal Fc Receptor (FcRn), Influence Rituximab Pharmacokinetics
- PMID: 40810921
- PMCID: PMC12618410
- DOI: 10.1007/s40262-025-01549-6
Immunoglobulin G Receptors (FcγR), in Addition to Target-Antigen and Neonatal Fc Receptor (FcRn), Influence Rituximab Pharmacokinetics
Abstract
Introduction: Rituximab, an anti-cluster of differentiation (CD)-20 monoclonal antibody, is used in the treatment of non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia, and rheumatoid arthritis. The pharmacokinetics of rituximab have been reported to be target mediated, but this alone may not fully explain the nonlinear decay of its concentrations over time.
Objective: This study aimed to explore the potential role of immunoglobulin (Fc gamma receptor; FcγR) and neonatal Fc receptor (FcRn) in the disposition of rituximab.
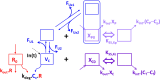
Methods: Concentration-time data from 108 patients with NHL, 118 with chronic lymphocytic leukemia, and 90 with rheumatoid arthritis were collected to refine a two-compartment population pharmacokinetic model with target-mediated drug disposition and irreversible binding approximation. Non-specific rituximab elimination was described using an intercompartment FcRn-mediated disposition model. Additionally, rituximab was assumed to bind to FcγR-expressing cells in both central and peripheral compartments; its disposition resulting from these mechanisms was described using quasi-steady-state interaction models.
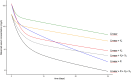
Results: The FcRn-mediated disposition model provided a satisfactory description of the data and was further improved by incorporating central and peripheral FcγR quasi-steady-state interaction models with steady-state dissociation constants estimated at 586 and 418 nM, respectively. CD19 cell count was related to target-mediated elimination rate constant (p = 1.7 × 10-8) and inversely related to non-specific elimination (assessed by estimated FcRn amount, p = 2.1 × 10-8). In patients with NHL, FcγR levels in central and peripheral compartments increased with baseline metabolic tumor volume (p = 7.0 × 10-6 and p = 5.0 × 10-28, respectively).
Conclusion: The pharmacokinetics of rituximab are mediated both by Fab (target) interactions and by FcγR and FcRn interactions.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study used rituximab pharmacokinetic data belonging to the Lymphoma Study Association (LYSA, France), the French Innovative Leukemia Organization (FILO, France), the Mayo Clinic (Rochester, MN, USA), and Tours University Hospital (Tours, France). The study in patients with DLBCL or follicular lymphoma was funded by GELA and GOELAMS groups and F. Hoffman-La Roche Ltd (Basel, Switzerland). The study in patients with CLL was funded by the FILO Group and Roche SAS (Neuilly, France). Measurements of rituximab serum concentrations in patients with CLL, DLBCL, follicular lymphoma (FL), or RA were carried out via the CePiBAc platform. CePiBAc was co-financed by the EU. Europe is committed to the region Centre with the European Regional Development Fund. CePiBAc was partly supported by the French Higher Education and Research ministry under the program ‘Investissements d’avenir’ grant agreement: LabEx MAbImprove ANR-10-LABX-53-01. Conflicts of Interest: Theodora Bejan-Angoulvant has no conflicts of interest to declare. She can be added to the lists of authors with no conflict of interest, for instance 'Céline Desvignes, Theodora Bejan-Angoulvant and Valérie Gouilleux-Gruart have no conflicts of interest to declare Ethics Approval: All studies were approved by the local ethics committee, and all patients provided written informed consent. Samples from the registered single-center cohort (University Hospital of Tours, France) with RA were primarily used for therapeutic drug monitoring routine, are part of a declared biological collection (N◦DC2016–2739), and conformed with the National Data Information and Freedom Commission. All data sets were anonymized before data analysis. Consent to Participate: Patients provided written informed consent, which included consent to participate. Consent for Publication: Patients provided written informed consent, which included consent for publication. Data Availability:: Data and material and code are available upon request to the corresponding author. Code Availability:: Code is available upon request to the corresponding author. Author Contributions: Céline Desvignes contributed to materials, participated in data management and reviewed the manuscript. Theodora Bejan-Angoulvant analyzed the data, interpreted the results and reviewed the manuscript.
Figures

References
-
- Bensalem A, Ternant D. Pharmacokinetic variability of therapeutic antibodies in humans: a comprehensive review of population pharmacokinetic modeling publications. Clin Pharmacokinet. 2021;59:857–74. - PubMed
-
- Petitcollin A, Bensalem A, Verdier MC, et al. Modelling of the time-varying pharmacokinetics of therapeutic monoclonal antibodies: a literature review. Clin Pharmacokinet. 2021;59:37–49. - PubMed
-
- Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–32. - PubMed
-
- Ternant D, Azzopardi N, Raoul W, et al. Influence of antigen mass on the pharmacokinetics of therapeutic antibodies in humans. Clin Pharmacokinet. 2019;58:169–87. - PubMed
-
- Bensalem A, Cartron G, Specks U, et al. The influence of underlying disease on rituximab pharmacokinetics may be explained by target-mediated drug disposition. Clin Pharmacokinet. 2022;61:423–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

