Photon and particle radiotherapy induce redundant modular chemotaxis of human lymphocytes
- PMID: 40811032
- PMCID: PMC12487834
- DOI: 10.1172/jci.insight.190149
Photon and particle radiotherapy induce redundant modular chemotaxis of human lymphocytes
Abstract
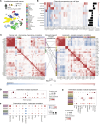
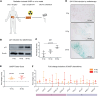
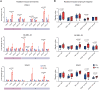
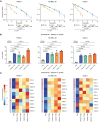
Radiotherapy triggers chemokine release and leukocyte infiltration in preclinical models through activation of the senescence-associated secretory phenotype (SASP). However, effects of irradiation on senescence and SASP in human tissue and in the context of particle radiotherapy remain unclear. Here, we analyzed chemokine patterns after radiotherapy of human pancreatic tumors and cancer cell lines. We show that irradiated tumor cells coexpressed SASP chemokines in defined modules. These chemokine modules correlated with infiltration of distinct leukocyte subtypes expressing cognate receptors. We developed a patient-derived pancreatic tumor explant system, which verified our identified radiation-induced chemokine modules. Chemokine modules were partially conserved in cancer cells in response to photon and particle irradiation, showing a dose-dependent plateau effect, and induced subsequent migration of NK and T cell populations. Hence, our work reveals redundant interactions of cancer cells and immune cells in human tissue, suggesting that targeting multiple chemokines is required to efficiently perturb leukocyte infiltration after photon or particle radiotherapy.
Keywords: Cellular senescence; Chemokines; Immunology; Oncology; Radiation therapy.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

