High-speed voltage imaging of action potentials in molecular layer interneurons reveals sensory-driven synchrony that augments movement
- PMID: 40811060
- PMCID: PMC12514754
- DOI: 10.1016/j.celrep.2025.116148
High-speed voltage imaging of action potentials in molecular layer interneurons reveals sensory-driven synchrony that augments movement
Abstract
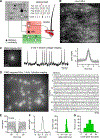
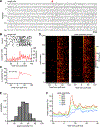
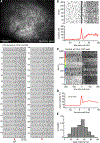
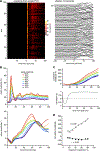
Testing whether the synchrony of action potential firing is a cerebellar coding mechanism requires simultaneous recording, with high temporal fidelity, from populations of identified neurons. Here, we used targeted one-photon voltage imaging at 2-4 kHz to record action potentials from groups of ∼10-300 molecular layer interneurons (MLIs) expressing a positively tuned, genetically encoded voltage indicator, FORCE1f or pAce. In awake resting mice, crus I MLIs fired brief (∼1-ms) spikes at 20-60 spikes/s. Sensory stimuli of air puffs to the whiskers evoked short-latency (<10 ms) increases in spiking probability. In most trials, >50% of MLIs fired synchronously with 4-ms temporal precision. The magnitude of puff-evoked whisks correlated tightly with the trial-by-trial percentage of synchrony. Brief optogenetic stimulation of MLIs was sufficient to induce and augment whisker protraction, whereas overriding MLI inhibition by stimulating target Purkinje cells reduced protractions, providing direct evidence that sensory-evoked spike synchrony can generate movement.
Keywords: CP: Neuroscience; Purkinje; cerebellum; parallel fiber; prediction; sensorimotor; whisker.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests F.S.-P. holds a US patent (#US9606100 B2) that encompasses the design of the FORCE1f indicator used in this article.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

