Platelets sequester extracellular DNA, capturing tumor-derived and free fetal DNA
- PMID: 40811534
- PMCID: PMC7618233
- DOI: 10.1126/science.adp3971
Platelets sequester extracellular DNA, capturing tumor-derived and free fetal DNA
Abstract
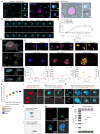
Platelets are anucleate blood cells vital for hemostasis and immunity. During cell death and aberrant mitosis, nucleated cells release DNA, resulting in "cell-free" DNA in plasma (cfDNA). An excess of cfDNA is deleterious. Given their ability to internalize pathogen-derived nucleic acids, we hypothesized that platelets may also clear endogenous cfDNA. We found that, despite lacking a nucleus, platelets contained a repertoire of DNA fragments mapping across the nuclear genome. We detected fetal DNA in maternal platelets and cancer-derived DNA in platelets from patients with premalignant and cancerous lesions. As current liquid biopsy approaches utilize platelet-depleted plasma, important genetic information contained within platelets is being missed. This study establishes a physiological role for platelets that has not previously been highlighted, with broad translational relevance.
Conflict of interest statement
BP, AJM, CGC, LM, and NS are listed as inventors on patent GB2203947.3 (CRUK Ref: CR/2021-069), submitted in 2022 by the University of Oxford, which covers the detection of disease-associated gene mutations in nucleic acids derived from purified platelets. BP, AJM, and LM are also inventors on patent P45651WO1 (GB2314848.9), filed in 2023 by the University of Oxford, relating to the recovery of cell-free nucleic acids from platelets. BP is a co-founder and major shareholder of Alethiomics, has received honoraria for speaking engagements and/or advisory work for Incyte, BMS, Constellation Therapeutics, GSK, Blueprint Medicines, Novartis and Alethiomics and received research funding from Incyte, Galecto and Alethiomics. AJM is a co-founder and shareholder of Alethiomics, has received honoraria/served on advisory boards for Alethiomics, Novartis, CTI, Gilead and Shire has received research funding from Alethiomics and Galecto. LM has consulted for Alethiomics. All other authors declare that they have no conflicts of interest.
Figures




Comment in
-
The ins and outs of circulating DNA.Science. 2025 Aug 14;389(6761):683-684. doi: 10.1126/science.aea0555. Epub 2025 Aug 14. Science. 2025. PMID: 40811562
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

